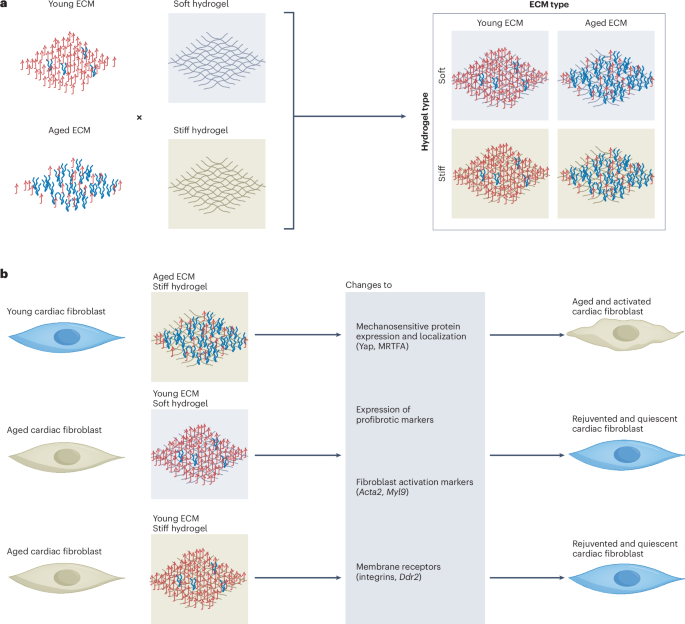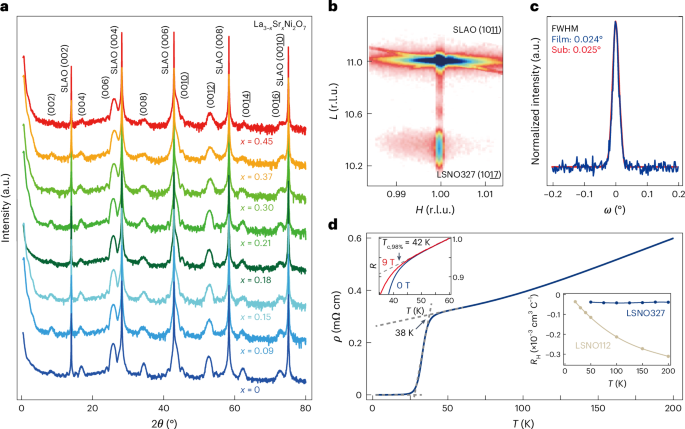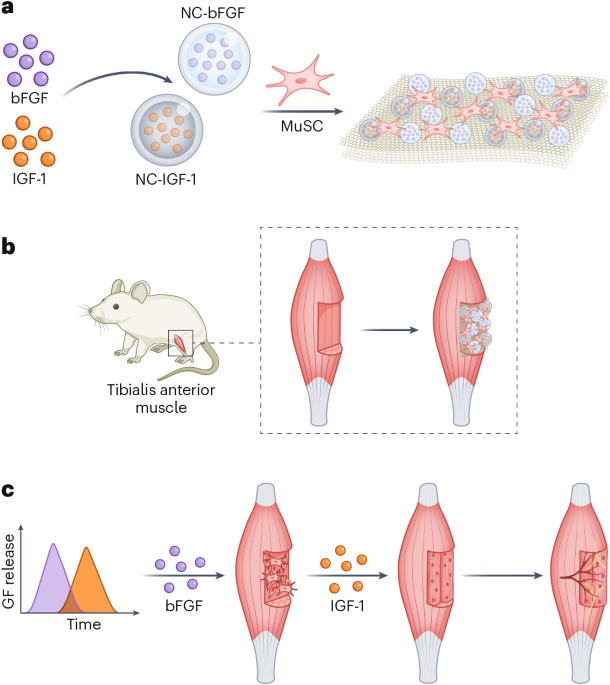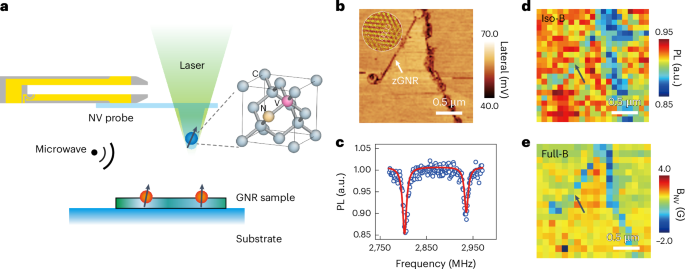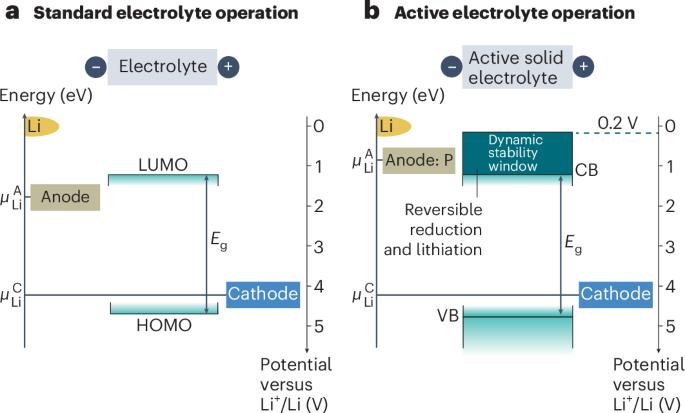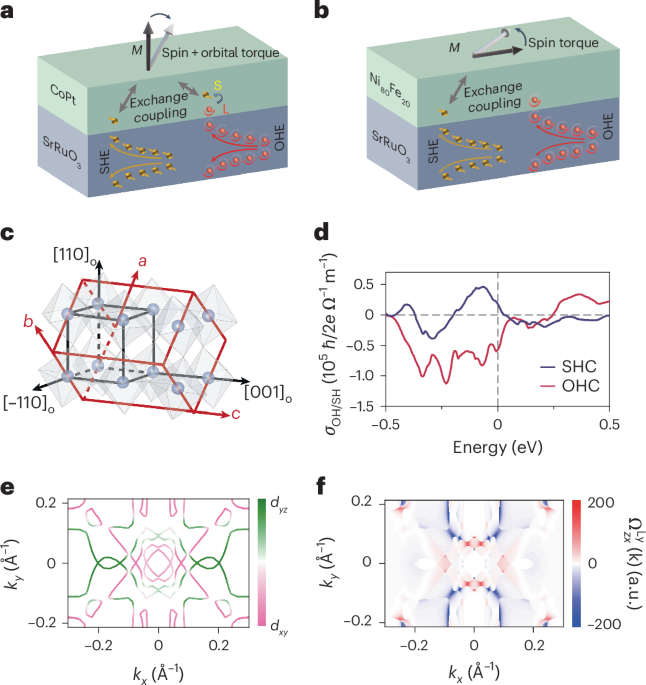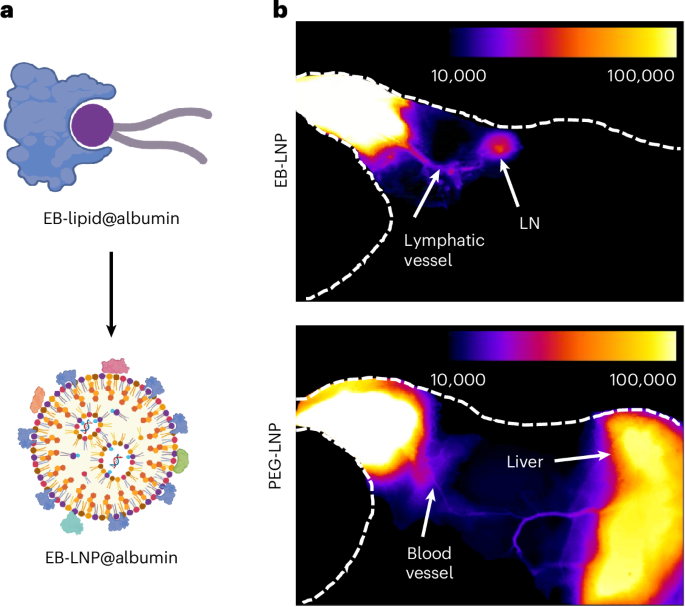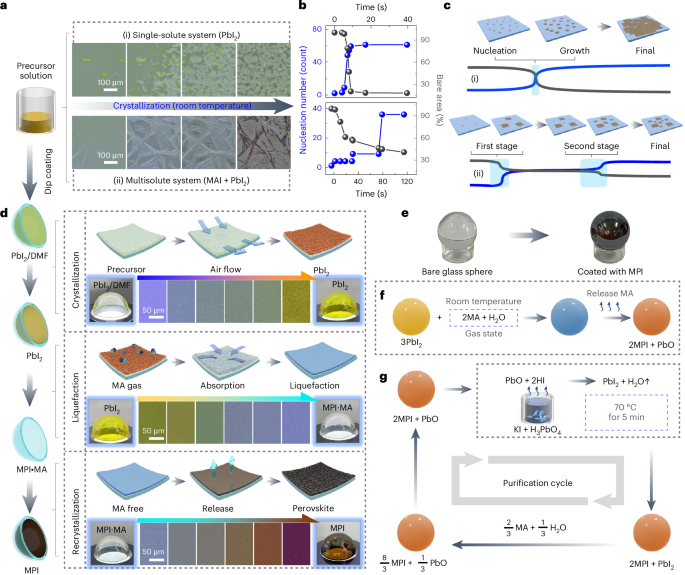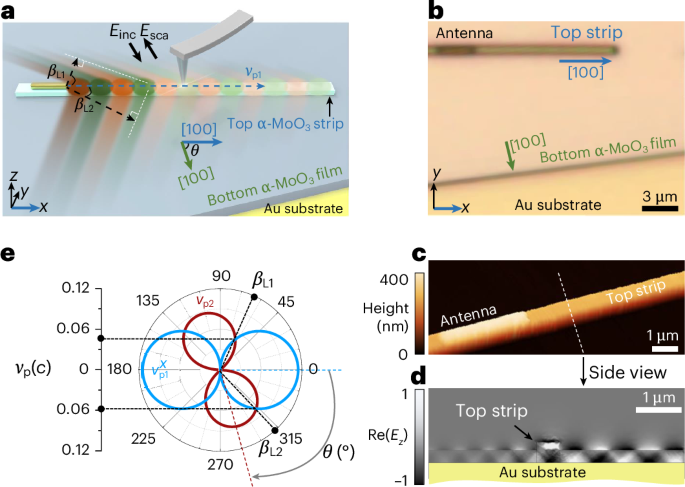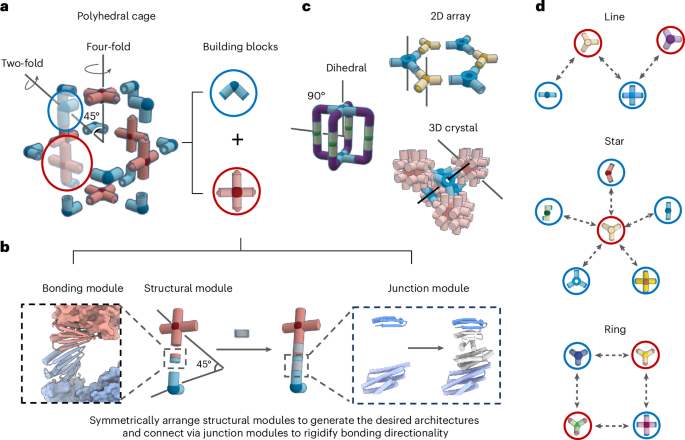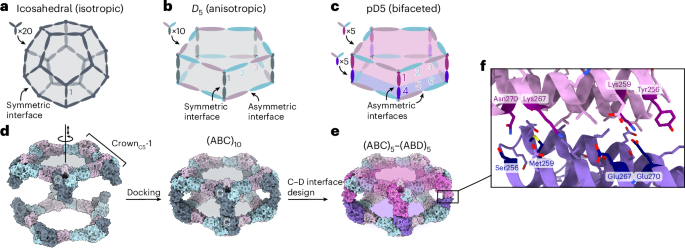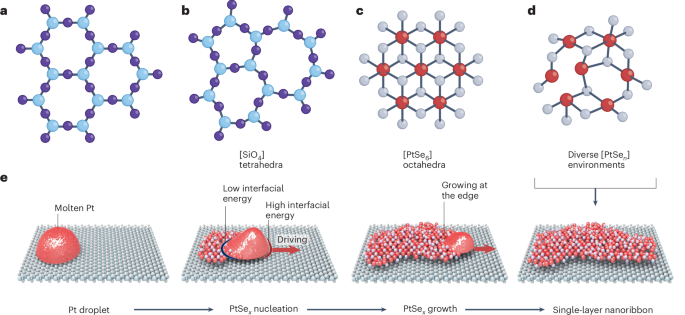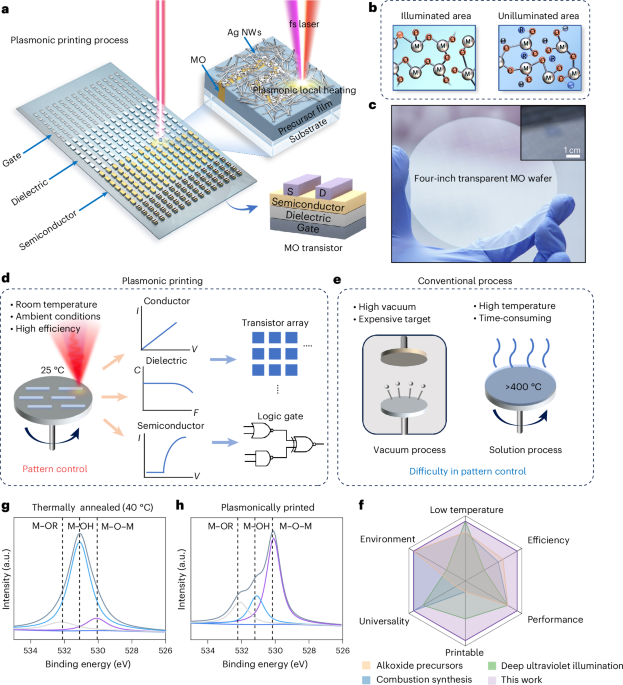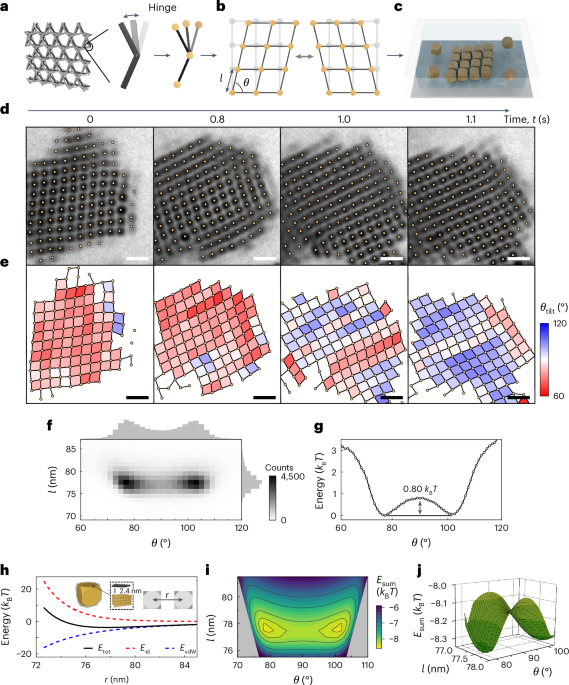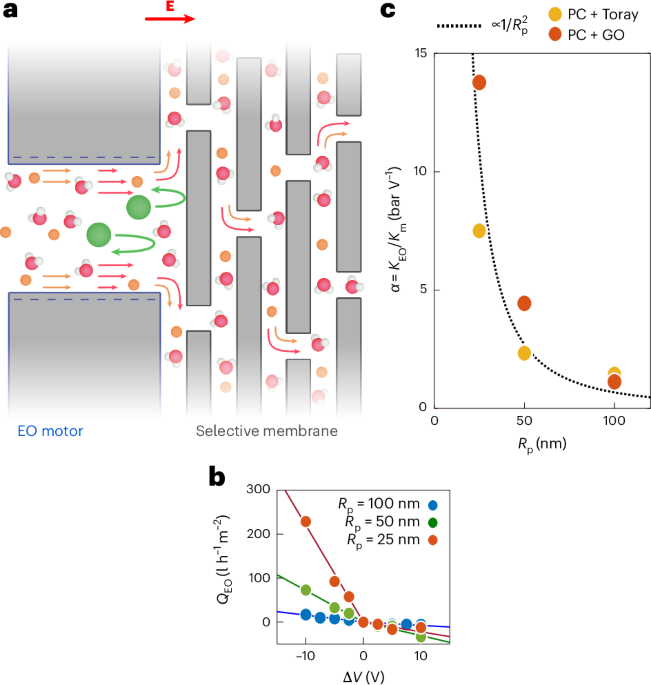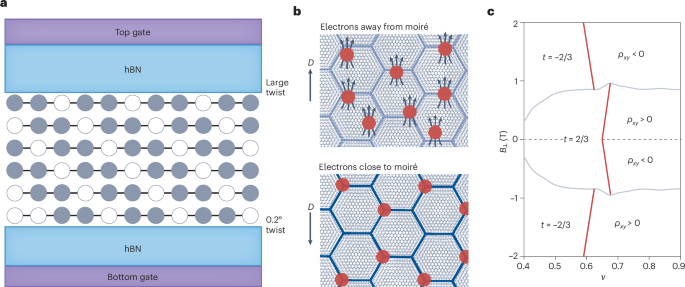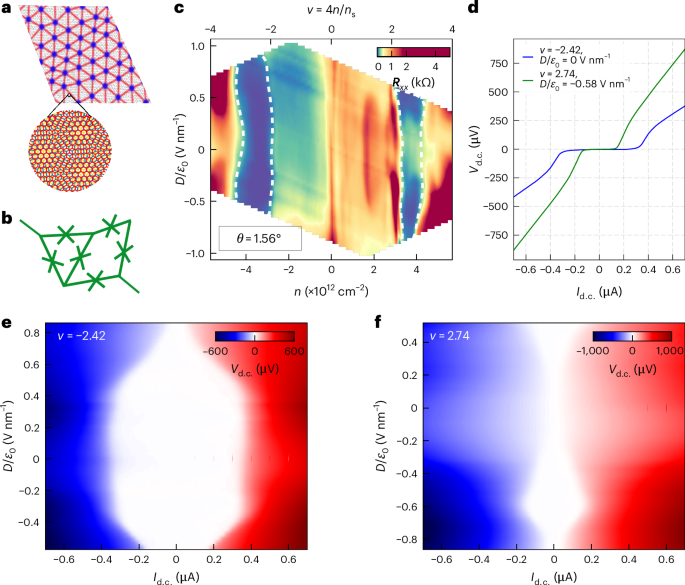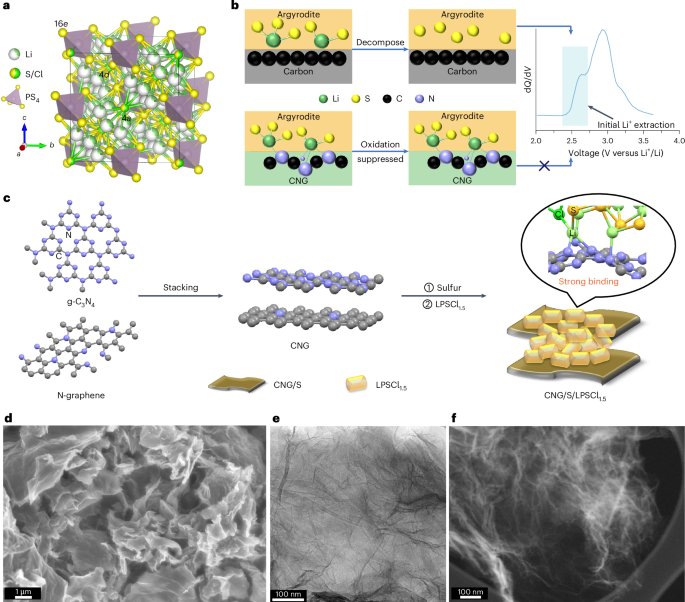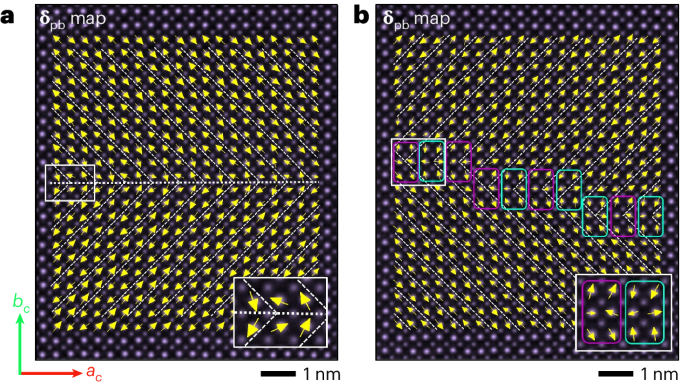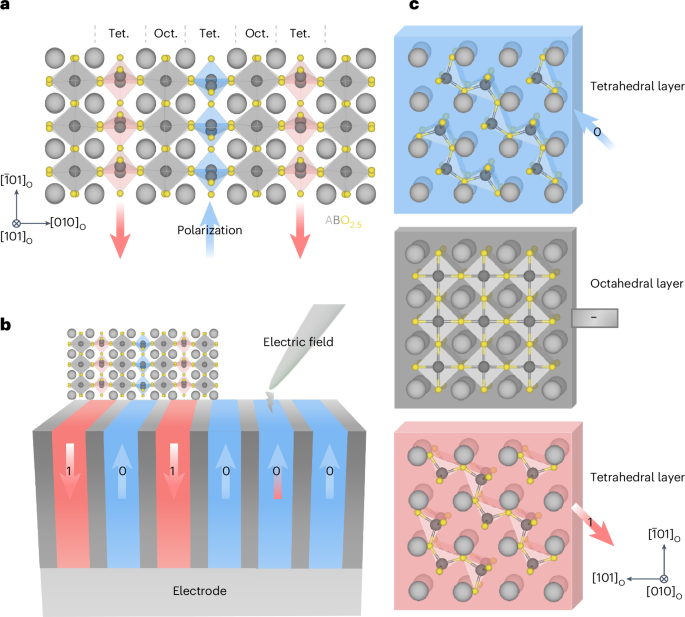
Manthiram, A. A reflection on lithium-ion battery cathode chemistry. Nat. Commun. 11, 1550 (2020).
Google Scholar
Yabuuchi, N. et al. P2-type Nax[Fe1/2Mn1/2]O2 made from Earth-abundant elements for rechargeable Na batteries. Nat. Mater. 11, 512–517 (2012).
Google Scholar
Saha, S. et al. Exploring the bottlenecks of anionic redox in Li-rich layered sulfides. Nat. Energy 4, 977–987 (2019).
Google Scholar
Wang, X. et al. Achieving a high-performance sodium-ion pouch cell by regulating intergrowth structures in a layered oxide cathode with anionic redox. Nat. Energy 9, 184–196 (2024).
Google Scholar
Xu, K., von Cresce, A. & Lee, U. Differentiating contributions to ‘ion transfer’ barrier from interphasial resistance and Li⁺ desolvation at electrolyte/graphite interface. Langmuir 26, 11538–11543 (2010).
Google Scholar
Besenhard, J. O. & Fritz, H. P. The electrochemistry of black carbons. Angew. Chem. Int. Ed. 22, 950–975 (1983).
Wagner, M. R., Albering, J. H., Moeller, K.-C., Besenhard, J. O. & Winter, M. XRD evidence for the electrochemical formation of Li⁺(PC)yCn− in PC-based electrolytes. Electrochem. Commun. 7, 947–952 (2005).
Google Scholar
Houdeville, R. G., Black, A. P., Ponrouch, A., Palacín, M. R. & Fauth, F. Operando synchrotron X-ray diffraction studies on TiS2: the effect of propylene carbonate on reduction mechanism. J. Electrochem. Soc. 168, 030514 (2021).
Google Scholar
Chung, G.-C. et al. Origin of graphite exfoliation: an investigation of the important role of solvent cointercalation. J. Electrochem. Soc. 147, 4391–4398 (2000).
Google Scholar
Park, J., Xu, Z.-L. & Kang, K. Solvated ion intercalation in graphite: sodium and beyond. Front. Chem. 8, 432 (2020).
Google Scholar
Guo, H., Elmanzalawy, M., Sivakumar, P. & Fleischmann, S. Unifying electrolyte formulation and electrode nanoconfinement design to enable new ion–solvent cointercalation chemistries. Energy Environ. Sci. 17, 2100–2116 (2024).
Google Scholar
Jache, B. & Adelhelm, P. Use of graphite as a highly reversible electrode with superior cycle life for sodium-ion batteries by making use of co-intercalation phenomena. Angew. Chem. Int. Ed. 53, 10169–10173 (2014).
Google Scholar
Kim, H. et al. Sodium storage behavior in natural graphite using ether-based electrolyte systems. Adv. Funct. Mater. 25, 534–541 (2015).
Google Scholar
Goktas, M. et al. Graphite as cointercalation electrode for sodium-ion batteries: electrode dynamics and the missing solid electrolyte interphase (SEI). Adv. Energy Mater. 8, 1702724 (2018).
Ferrero, G. A. et al. Solvent co-intercalation reactions for batteries and beyond. Chem. Rev. 125, 3401–3439 (2025).
Google Scholar
Jache, B., Binder, J. O., Abe, T. & Adelhelm, P. A comparative study on the impact of different glymes and their derivatives as electrolyte solvents for graphite co-intercalation electrodes in lithium-ion and sodium-ion batteries. Phys. Chem. Chem. Phys. 18, 14299–14316 (2016).
Google Scholar
Abe, T., Fukuda, H., Iriyama, Y. & Ogumi, Z. Solvated Li-ion transfer at interface between graphite and electrolyte. J. Electrochem. Soc. 151, A1120 (2004).
Google Scholar
Alvarez et al. Co-intercalation batteries (CoIBs): role of TiS2 as electrode for storing solvated Na ions. Adv. Energy Mater. 12, 2202377 (2022).
Jung, S. C., Kang, Y.-J. & Han, Y.-K. Origin of excellent rate and cycle performance of Na⁺-solvent cointercalated graphite vs. poor performance of Li⁺-solvent case. Nano Energy 34, 456–462 (2017).
Google Scholar
Leifer, N., Greenstein, M. F., Mor, A., Aurbach, D. & Goobes, G. NMR-detected dynamics of sodium co-intercalation with diglyme solvent molecules in graphite anodes linked to prolonged cycling. J. Phys. Chem. C 122, 21172–21184 (2018).
Google Scholar
McKinnon, W. R. & Dahn, J. R. How to reduce the cointercalation of propylene carbonate in LixZrS2 and other layered compounds. J. Electrochem. Soc. 132, 364–366 (1985).
Google Scholar
Park, J., Kim, S. J., Lim, K., Cho, J. & Kang, K. Reconfiguring sodium intercalation process of TiS2 electrode for sodium-ion batteries by a partial solvent cointercalation. ACS Energy Lett. 7, 3718–3726 (2022).
Google Scholar
Tchitchekova, D. S. et al. Electrochemical intercalation of calcium and magnesium in TiS2: fundamental studies related to multivalent battery applications. Chem. Mater. 30, 847–856 (2018).
Google Scholar
Åvall, G. et al. In situ pore formation in graphite through solvent co-intercalation: a new model for the formation of ternary graphite intercalation compounds bridging batteries and supercapacitors. Adv. Energy Mater. 13, 2301944 (2023).
Escher, I., Hahn, M., Ferrero, G. A. & Adelhelm, P. A practical guide for using electrochemical dilatometry as operando tool in battery and supercapacitor research. Energy Technol. 10, 2101120 (2022).
Palaniselvam, T. et al. Sodium storage and electrode dynamics of tin–carbon composite electrodes from bulk precursors for sodium-ion batteries. Adv. Funct. Mater. 29, 1900790 (2019).
Nayak, P. K. et al. Investigation of Li1.17Ni0.20Mn0.53Co0.10O2 as an interesting Li- and Mn-rich layered oxide cathode material through electrochemistry, microscopy, and in situ electrochemical dilatometry. ChemElectroChem 6, 2812–2819 (2019).
Google Scholar
Spingler, F. B., Kücher, S., Phillips, R., Moyassari, E. & Jossen, A. Electrochemically stable in situ dilatometry of NMC, NCA and graphite electrodes for lithium-ion cells compared to XRD measurements. J. Electrochem. Soc. 168, 040515 (2021).
Google Scholar
Escher, I., Freytag, A. I., Lopez del Amo, J. M. & Adelhelm, P. Solid-state NMR study on the structure and dynamics of graphite electrodes in sodium-ion batteries with solvent co-intercalation. Batter. Supercaps 6, e202200421 (2023).
Google Scholar
Pell, A. J., Pintacuda, G. & Grey, C. P. Paramagnetic NMR in solution and the solid state. Prog. Nucl. Magn. Reson. Spectrosc. 111, 1–271 (2019).
Google Scholar
Gotoh, K. et al. Structure and dynamic behavior of sodium–diglyme complex in the graphite anode of sodium ion battery by 2H nuclear magnetic resonance. J. Phys. Chem. C 120, 28152–28156 (2016).
Google Scholar
Yoon, G., Kim, H., Park, I. & Kang, K. Conditions for reversible Na intercalation in graphite: theoretical studies on the interplay among guest ions, solvent, and graphite host. Adv. Energy Mater. 7, 1601519 (2017).
Xu, K. Electrolytes, Interfaces and Interphases: Fundamentals and Applications in Batteries (Royal Society of Chemistry, 2023).
Chung, G.-C., Kim, H.-J., Jun, S.-H. & Kim, M.-H. New cyclic carbonate solvent for lithium ion batteries: trans-2,3-butylene carbonate. Electrochem. Commun. 1, 493–496 (1999).
Google Scholar
Xu, K. Electrolytes and interphases in Li-ion batteries and beyond. Chem. Rev. 114, 11503–11618 (2014).
Google Scholar
Wang, T. et al. Anionic redox reaction in layered NaCr2/3Ti1/3S2 through electron holes formation and dimerization of S–S. Nat. Commun. 10, 4458 (2019).
Google Scholar
Wang, T. et al. Anomalous redox features induced by strong covalency in layered NaTi1−yVyS2 cathodes for Na-ion batteries. Angew. Chem. Int. Ed. 61, e202205444 (2022).
Google Scholar
Bianchini, M. et al. The interplay between thermodynamics and kinetics in the solid-state synthesis of layered oxides. Nat. Mater. 19, 1088–1095 (2020).
Google Scholar
Vassilaras, P., Ma, X., Li, X. & Ceder, G. Electrochemical properties of monoclinic NaNiO2. J. Electrochem. Soc. 160, A207–A211 (2013).
Google Scholar
Dippel, A.-C. et al. Beamline P02.1 at PETRA III for high-resolution and high-energy powder diffraction. J. Synchrotron Radiat. 22, 675–687 (2015).
Google Scholar
Kieffer, J., Valls, V., Blanc, N. & Hennig, C. New tools for calibrating diffraction setups. J. Synchrotron Radiat. 27, 558–566 (2020).
Google Scholar
Toby, B. H. & Von Dreele, R. B. GSAS-II: the genesis of a modern open-source all-purpose crystallography software package. J. Appl. Crystallogr. 46, 544–549 (2013).
Google Scholar
Frisch, M. J., et al. Gaussian 16 Revision C.01 (2016).
Becke, A. D. A new mixing of Hartree–Fock and local density-functional theories. J. Chem. Phys. 98, 1372–1377 (1993).
Google Scholar
Becke, A. D. Density-functional exchange-energy approximation with correct asymptotic behavior. Phys. Rev. A 38, 3098–3100 (1988).
Google Scholar
Lee, C., Yang, W. & Parr, R. G. Development of the Colle–Salvetti correlation-energy formula into a functional of the electron density. Phys. Rev. B 37, 785–789 (1988).
Google Scholar
Marenich, A. V., Cramer, C. J. & Truhlar, D. G. Universal solvation model based on solute electron density and on a continuum model of the solvent defined by the bulk dielectric constant and atomic surface tensions. J. Phys. Chem. B 113, 6378–6396 (2009).
Google Scholar
Perdew, J. P., Burke, K. & Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 77, 3865–3868 (1996).
Google Scholar
Kresse, G. & Furthmüller, J. Efficiency of ab-initio total energy calculations for metals and semiconductors using a plane-wave basis set. Comput. Mater. Sci. 6, 15–50 (1996).
Google Scholar
Kresse, G. & Furthmüller, J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B 54, 11169–11186 (1996).
Google Scholar
Kresse, G., Furthmüller, J. & Hafner, J. Theory of the crystal structures of selenium and tellurium: the effect of generalized-gradient corrections to the local-density approximation. Phys. Rev. B 50, 13181–13185 (1994).
Google Scholar
Kresse, G. & Hafner, J. Ab initio molecular dynamics for liquid metals. Phys. Rev. B 47, 558–561 (1993).
Google Scholar
Kresse, G. & Joubert, D. From ultrasoft pseudopotentials to the projector augmented-wave method. Phys. Rev. B 59, 1758–1775 (1999).
Google Scholar
Zagorac, D., Müller, H., Ruehl, S., Zagorac, J. & Rehme, S. Recent developments in the Inorganic Crystal Structure Database: theoretical crystal structure data and related features. J. Appl. Crystallogr. 52, 918–925 (2019).
Google Scholar
Holleman, A. F., Wiberg, E. & Wiberg, N. in Lehrbuch der anorganischen Chemie 2035–2038 (1985).
Sun, Y., Åvall, G. & Adelhelm, P. Solvent co-intercalation in layered cathode active materials for sodium-ion batteries. figshare https://doi.org/10.6084/m9.figshare.29143679 (2025).