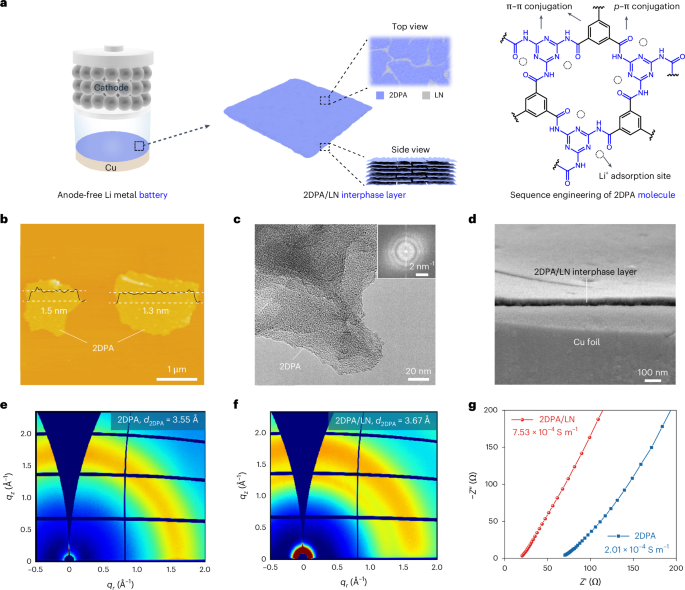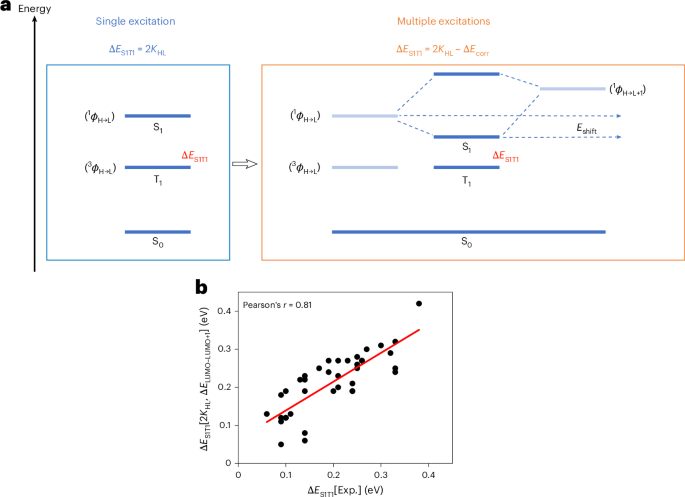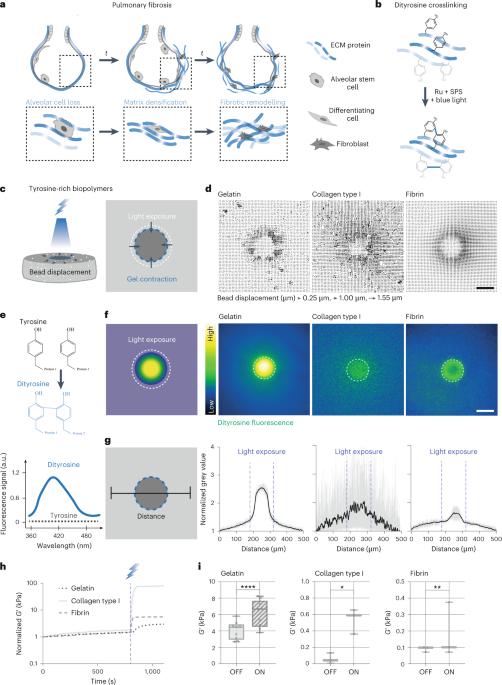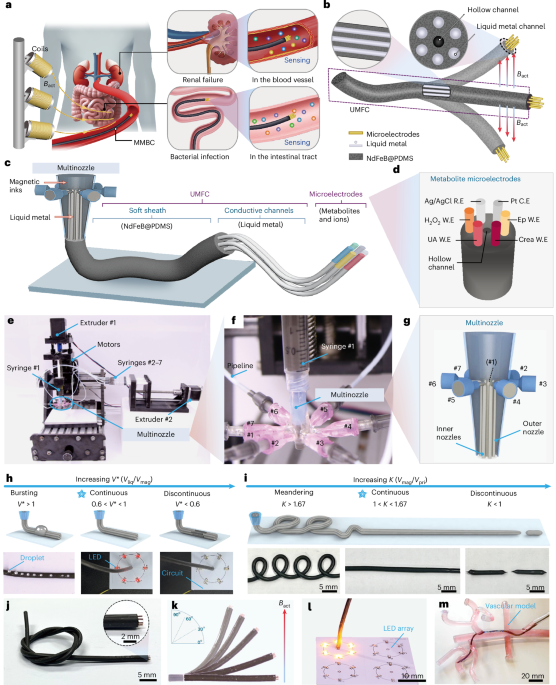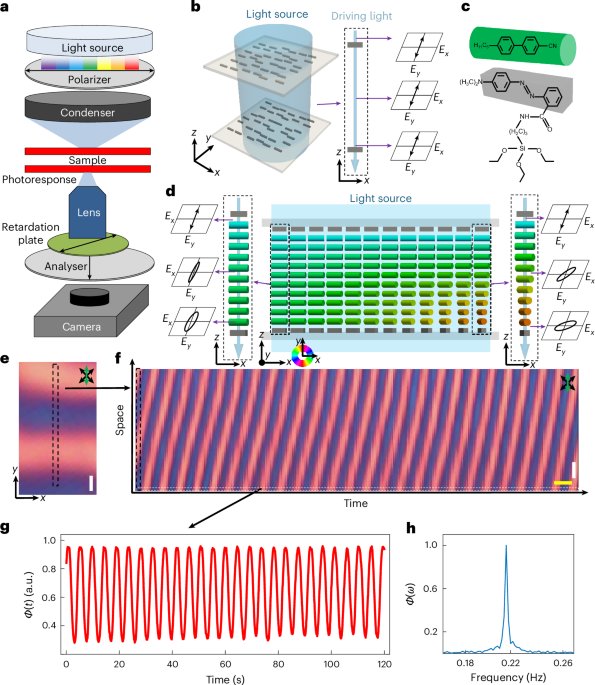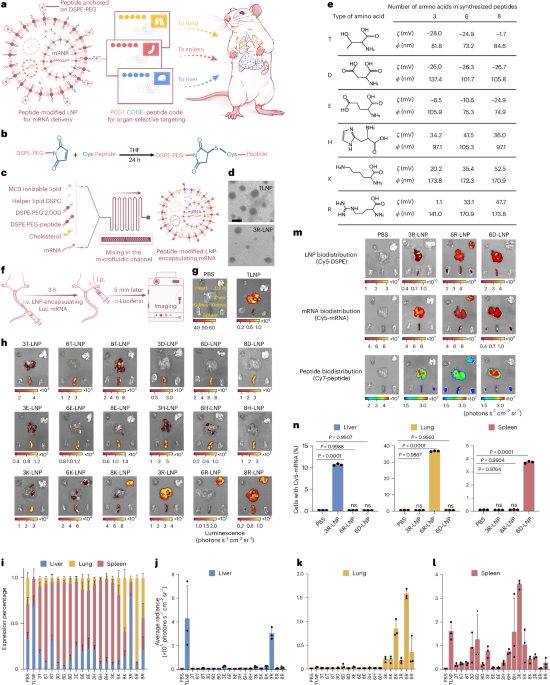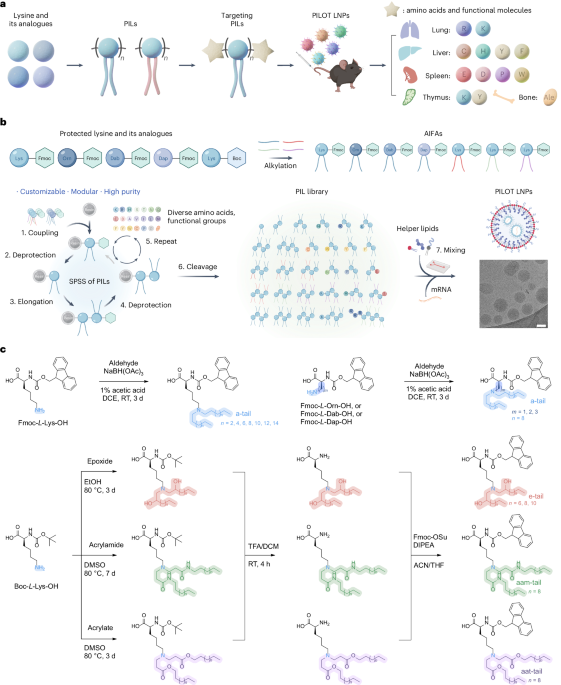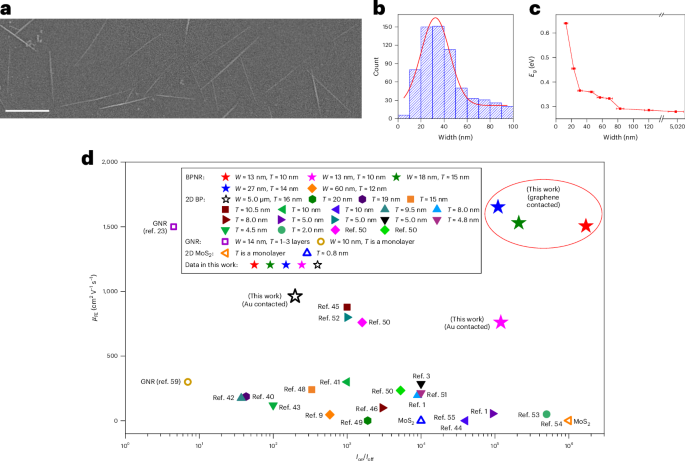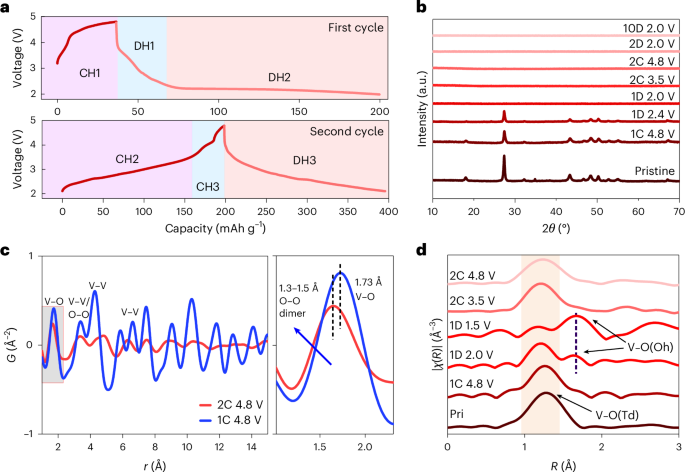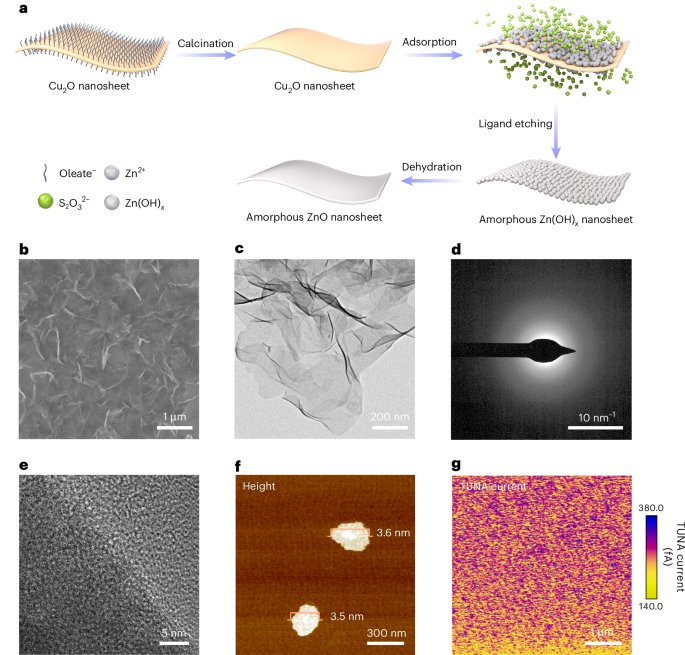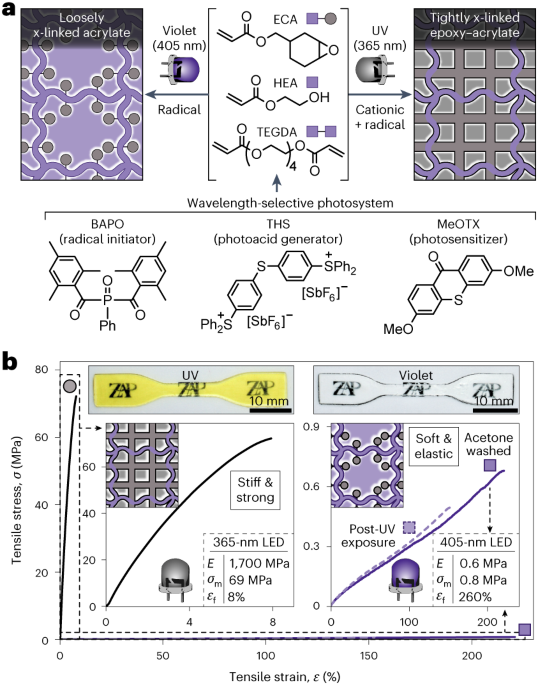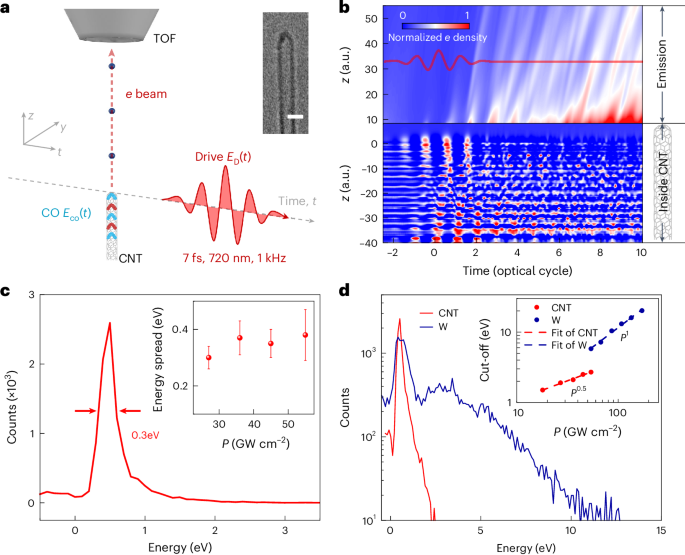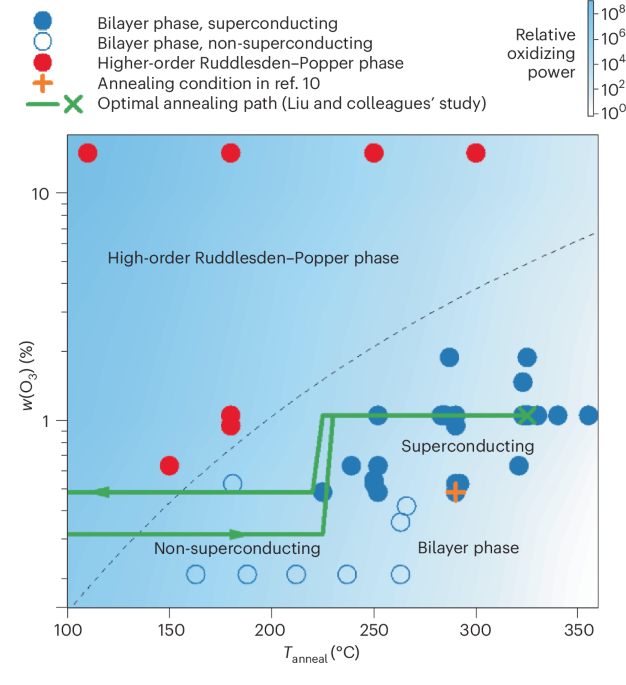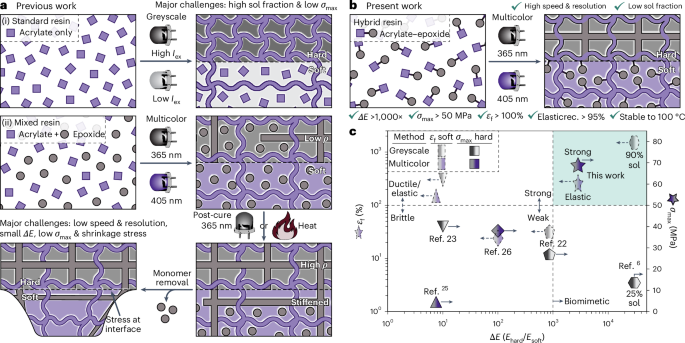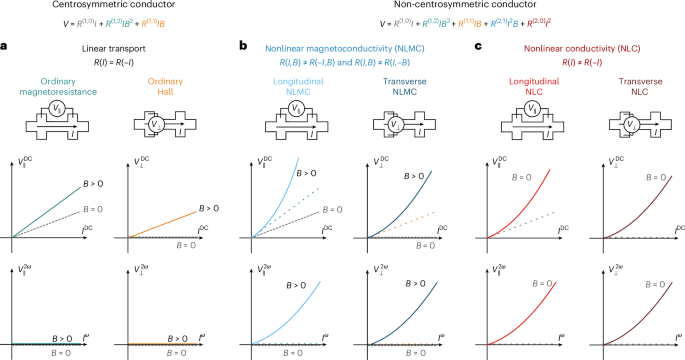
Robin, P., Kavokine, N. & Bocquet, L. Modeling of emergent memory and voltage spiking in ionic transport through angstrom-scale slits. Science 373, 687–691 (2021).
Google Scholar
Robin, P. et al. Long-term memory and synapse-like dynamics in two-dimensional nanofluidic channels. Science 379, 161–167 (2023).
Google Scholar
Zhang, Z. et al. Perovskite nickelates as electric-field sensors in salt water. Nature 553, 68–72 (2018).
Google Scholar
Jayachandran, D. et al. A low-power biomimetic collision detector based on an in-memory molybdenum disulfide photodetector. Nat. Electron. 3, 646–655 (2020).
Ahmed, D. et al. Bioinspired acousto-magnetic microswarm robots with upstream motility. Nat. Mach. Intell. 3, 116–124 (2021).
Debanne, D., Inglebert, Y. & Russier, M. Plasticity of intrinsic neuronal excitability. Curr. Opin. Neurobiol. 54, 73–82 (2019).
Google Scholar
Chun, H. & Chung, T. D. Iontronics. Annu. Rev. Anal. Chem. 8, 441–462 (2015).
Bisri, S. Z., Shimizu, S., Nakano, M. & Iwasa, Y. Endeavor of iontronics: from fundamentals to applications of ion‐controlled electronics. Adv. Mater. 29, 1607054 (2017).
Van Doremaele, E. R. W., Ji, X., Rivnay, J. & Van De Burgt, Y. A retrainable neuromorphic biosensor for on-chip learning and classification. Nat. Electron. 6, 792–800 (2023).
Van De Burgt, Y. et al. A non-volatile organic electrochemical device as a low-voltage artificial synapse for neuromorphic computing. Nat. Mater. 16, 414–418 (2017).
Xie, K. et al. Organic electrochemical transistor arrays for real-time mapping of evoked neurotransmitter release in vivo. eLife 9, e50345 (2020).
Google Scholar
Zhang, P. et al. Nanochannel-based transport in an interfacial memristor can emulate the analog weight modulation of synapses. Nano Lett. 19, 4279–4286 (2019).
Google Scholar
Xiong, T. et al. Neuromorphic functions with a polyelectrolyte-confined fluidic memristor. Science 379, 156–161 (2023).
Google Scholar
Ding, K. et al. Phase-change heterostructure enables ultralow noise and drift for memory operation. Science 366, 210–215 (2019).
Google Scholar
Shi, J., Ha, S. D., Zhou, Y., Schoofs, F. & Ramanathan, S. A correlated nickelate synaptic transistor. Nat. Commun. 4, 2676 (2013).
Park, T. J. et al. Complex oxides for brain‐inspired computing: a review. Adv. Mater. 35, 2203352 (2023).
Google Scholar
Jeong, J. et al. Suppression of metal-insulator transition in VO2 by electric field-induced oxygen vacancy formation. Science 339, 1402–1405 (2013).
Google Scholar
Zhou, Y. et al. Control of emergent properties at a correlated oxide interface with graphene. Nano Lett. 15, 1627–1634 (2015).
Google Scholar
Wang, S. et al. An organic electrochemical transistor for multi-modal sensing, memory and processing. Nat. Electron. 6, 281–291 (2023).
Google Scholar
Vahl, A. et al. Concept and modelling of memsensors as two terminal devices with enhanced capabilities in neuromorphic engineering. Sci. Rep. 9, 4361 (2019).
Suzuki, H. et al. Functional asymmetry in Caenorhabditis elegans taste neurons and its computational role in chemotaxis. Nature 454, 114–117 (2008).
Google Scholar
Appleby, P. A. A model of chemotaxis and associative learning in C. elegans. Biol. Cybern. 106, 373–387 (2012).
Ferkey, D. M., Sengupta, P. & L’Etoile, N. D. Chemosensory signal transduction in Caenorhabditis elegans. Genetics 217, iyab004 (2021).
Dekkers, M. P. J. et al. Plasticity in gustatory and nociceptive neurons controls decision making in C. elegans salt navigation. Commun. Biol. 4, 1053 (2021).
Google Scholar
Chen, Y. et al. Non-catalytic hydrogenation of VO2 in acid solution. Nat. Commun. 9, 818 (2018).
Queirós, L. et al. Overview of chemotaxis behavior assays in Caenorhabditis elegans. Curr. Protoc. 1, e120 (2021).
Park, C. et al. Roles of the ClC chloride channel CLH-1 in food-associated salt chemotaxis behavior of C. elegans. eLife 10, e55701 (2021).
Google Scholar
Jiang, L., Mo, H. & Tian, P. A bacterial chemotaxis-inspired coordination strategy for coverage and aggregation of swarm robots. Appl. Sci. 11, 1347 (2021).
Google Scholar
Nurzaman, S. G., Matsumoto, Y., Nakamura, Y., Koizumi, S. & Ishiguro, H. ‘Yuragi’-based adaptive mobile robot search with and without gradient sensing: from bacterial chemotaxis to a Levy walk. Adv. Robot. 25, 2019–2037 (2011).
Kunitomo, H. et al. Concentration memory-dependent synaptic plasticity of a taste circuit regulates salt concentration chemotaxis in Caenorhabditis elegans. Nat. Commun. 4, 2210 (2013).
Qiao, R. et al. High-efficiency in situ resonant inelastic X-ray scattering (iRIXS) endstation at the Advanced Light Source. Rev. Sci. Instrum. 88, 033106 (2017).
Kurmaev, E. Z. et al. Oxygen X-ray emission and absorption spectra as a probe of the electronic structure of strongly correlated oxides. Phys. Rev. B 77, 165127 (2008).
Stępień, J., Sikora, M., Kapusta, C., Pomykalska, D. & Bućko, M. M. Determination of oxygen vacancy limit in Mn substituted yttria stabilized zirconia. J. Appl. Phys. 123, 185108 (2018).
Hazarika, R. & Kalita, B. Effect of oxygen vacancy defects on electronic and optical properties of MgO monolayers: first principles study. Mater. Sci. Eng. B 286, 115974 (2022).
Google Scholar
Kurbatov, A. P. et al. Chemical oxidation of LiFePO4 in aqueous medium as a method for studying kinetics of delithiation. Russ. J. Electrochem. 54, 225–233 (2018).
Google Scholar
Weichert, K. et al. Phase boundary propagation in large LiFePO4 single crystals on delithiation. J. Am. Chem. Soc. 134, 2988–2992 (2012).
Google Scholar
Tamura, H., Mita, K., Tanaka, A. & Ito, M. Mechanism of hydroxylation of metal oxide surfaces. J. Colloid Interface Sci. 243, 202–207 (2001).
Google Scholar
Anderson, M. & Rubin, A. Adsorption of inorganics at solid-liquid interfaces. Soil Sci. 133, 257–258 (1982).
Zenkin, S., Kos, Š. & Musil, J. Hydrophobicity of thin films of compounds of low-electronegativity metals. J. Am. Ceram. Soc. 97, 2713–2717 (2014).
Google Scholar
Xi, Y., Qi, Y., Mao, Z., Yang, Z. & Zhang, J. Surface hydrophobic modification of TiO2 and its application to preparing PMMA/TiO2 composite cool material with improved hydrophobicity and anti-icing property. Constr. Build. Mater. 266, 120916 (2021).
Google Scholar
Luo, S., Huo, J. C., Lei, H. & Deng, J. Study of the hydrophobic modification of chromium sesquioxide with lauric acid. Adv. Mater. Res. 535–537, 1586–1590 (2012).
Bischoff, M., Biriukov, D., Předota, M., Roke, S. & Marchioro, A. Surface potential and interfacial water order at the amorphous TiO2 nanoparticle/aqueous interface. J. Phys. Chem. C 124, 10961–10974 (2020).
Google Scholar
Lützenkirchen, J. Comparison of 1-pK and 2-pK versions of surface complexation theory by the goodness of fit in describing surface charge data of (hydr)oxides. Environ. Sci. Technol. 32, 3149–3154 (1998).
Li, M. et al. Relationship between surface hydroxyl complexation and equi-acidity point pH of MnO2 and its adsorption for Co2+ and Ni2+. ACS Omega 7, 9602–9613 (2022).
Google Scholar
Kitagawa, Y., Yamaguchi, S. & Yorozu, Y. Effects of sodium chloride concentrations on zeta potentials of clay minerals estimated by an electrokinetic sonic amplitude method. Clay Sci. 12, 91–96 (2003).
Google Scholar
Vilasau, J. et al. Stability of oil-in-water paraffin emulsions prepared in a mixed ionic/nonionic surfactant system. Colloids Surf. A Physicochem. Eng. Asp. 389, 222–229 (2011).
Google Scholar
Behrens, S. H. & Grier, D. G. The charge of glass and silica surfaces. J. Chem. Phys. 115, 6716–6721 (2001).
Google Scholar
Jain, A. et al. Commentary: The Materials Project: a materials genome approach to accelerating materials innovation. APL Mater. 1, 011002 (2013).
Kluczka, J. A review on the recovery and separation of gallium and indium from waste. Resources 13, 35 (2024).
Baes, C. F. & Mesmer, R. S. The hydrolysis of cations. Ber. Bunsenges. Phys. Chem. 81, 245–246 (1977).
Brown, M. A., Goel, A. & Abbas, Z. Effect of electrolyte concentration on the Stern layer thickness at a charged interface. Angew. Chem. Int. Ed. 55, 3790–3794 (2016).
Li, Y. et al. Fluid-enhanced surface diffusion controls intraparticle phase transformations. Nat. Mater. 17, 915–922 (2018).
Google Scholar
Bard, A. J. & Faulkner, L. R. Electrochemical Methods: Fundamentals and Applications (Wiley, 2001).
Turner, B. F. & Fein, J. B. Protofit: a program for determining surface protonation constants from titration data. Comput. Geosci. 32, 1344–1356 (2006).
Google Scholar
Piasecki, W. 1pK and 2pK protonation models in the theoretical description of simple ion adsorption at the oxide/electrolyte interface: a comparative study of the predicted and observed enthalpic effects accompanying adsorption of simple ions. Langmuir 18, 4809–4818 (2002).
Google Scholar
Schmickler, W. & Santos, E. in Interfacial Electrochemistry (eds Schmickler, W. & Santos, E.) 177–193 (Springer, 2010).
Kresse, G. & Hafner, J. Ab initio molecular dynamics for liquid metals. Phys. Rev. B 47, 558–561 (1993).
Google Scholar
Kresse, G. & Furthmüller, J. Efficiency of ab-initio total energy calculations for metals and semiconductors using a plane-wave basis set. Comput. Mater. Sci. 6, 15–50 (1996).
Google Scholar
Kresse, G. & Furthmüller, J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B 54, 11169–11186 (1996).
Google Scholar
Perdew, J. P., Burke, K. & Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 77, 3865–3868 (1996).
Google Scholar
Kresse, G. & Joubert, D. From ultrasoft pseudopotentials to the projector augmented-wave method. Phys. Rev. B 59, 1758–1775 (1999).
Google Scholar
Dudarev, S. L., Botton, G. A., Savrasov, S. Y., Humphreys, C. J. & Sutton, A. P. Electron-energy-loss spectra and the structural stability of nickel oxide: an LSDA+U study. Phys. Rev. B 57, 1505–1509 (1998).
Google Scholar
Kylänpää, I. et al. Accuracy of ab initio electron correlation and electron densities in vanadium dioxide. Phys. Rev. Mater. 1, 065408 (2017).
Stahl, B. & Bredow, T. Critical assessment of the DFT+U approach for the prediction of vanadium dioxide properties. J. Comput. Chem. 41, 258–265 (2020).
Google Scholar
Srivastava, S., Uberuaga, B. P. & Asta, M. Density functional theory study of local environment effects on oxygen vacancy properties in magnetite. J. Phys. Chem. C 127, 17460–17472 (2023).
Google Scholar
Freysoldt, C. et al. First-principles calculations for point defects in solids. Rev. Mod. Phys. 86, 253–305 (2014).
Freysoldt, C., Neugebauer, J. & Van De Walle, C. G. Fully ab initio finite-size corrections for charged-defect supercell calculations. Phys. Rev. Lett. 102, 016402 (2009).
Freysoldt, C., Neugebauer, J. & Van De Walle, C. G. Electrostatic interactions between charged defects in supercells. Phys. Status Solidi B 248, 1067–1076 (2011).
Google Scholar
Kumagai, Y. & Oba, F. Electrostatics-based finite-size corrections for first-principles point defect calculations. Phys. Rev. B 89, 195205 (2014).
Gonze, X. & Lee, C. Dynamical matrices, Born effective charges, dielectric permittivity tensors, and interatomic force constants from density-functional perturbation theory. Phys. Rev. B 55, 10355–10368 (1997).
Google Scholar
Liu, B., Xiao, H., Zhang, Y., Aidhy, D. S. & Weber, W. J. Investigation of oxygen point defects in cubic ZrO2 by density functional theory. Comput. Mater. Sci. 92, 22–27 (2014).
Google Scholar
Bratsch, S. G. Standard electrode potentials and temperature coefficients in water at 298.15 K. J. Phys. Chem. Ref. Data 18, 1–21 (1989).
Google Scholar
Guo, R. Data for ‘Mem-sensing by surface ion migration within Debye length’. Zenodo https://doi.org/10.5281/zenodo.15758758 (2025).