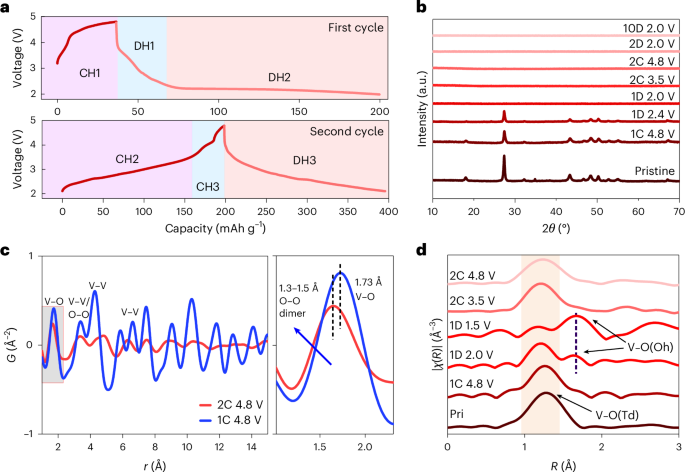
Iwahara, H., Esaka, T., Uchida, H. & Maeda, N. Proton conduction in sintered oxides and its application to steam electrolysis for hydrogen production. Solid State Ion. 3–4, 359–363 (1981).
Yamazaki, Y., Hernandez-Sanchez, R. & Haile, S. M. High total proton conductivity in large-grained yttrium-doped barium zirconate. Chem. Mater. 21, 2755–2762 (2009).
Google Scholar
Hyodo, J., Kitabayashi, K., Hoshino, K., Okuyama, Y. & Yamazaki, Y. Fast and stable proton conduction in heavily scandium-doped polycrystalline barium zirconate at intermediate temperatures. Adv. Energy Mater. 10, 2000213 (2020).
Google Scholar
Kanno, R. & Murayama, M. Lithium ionic conductor thio-LISICON. J. Electrochem. Soc. 148, A742–A746 (2001).
Google Scholar
Kato, Y. et al. High-power all-solid-state batteries using sulfide superionic conductors. Nat. Energy 1, 16030 (2016).
Google Scholar
Duan, C. et al. Readily processed protonic ceramic fuel cells with high performance at low temperatures. Science 349, 1321–1326 (2015).
Google Scholar
Morejudo, S. H. et al. Direct conversion of methane to aromatics in a catalytic co-ionic membrane reactor. Science 353, 563–566 (2016).
Google Scholar
Wang, J., Wasmu, S. & Savinel, R. F. Evaluation of ethanol, 1-propanol, and 2-propanol in a direct oxidation polymer-electrolyte fuel cell. J. Electrochem. Soc. 142, 4218–4224 (1995).
Google Scholar
Fukui, K., Iimura, S., Iskandarov, A., Tada, T. & Hosono, H. Room-temperature fast H− conduction in oxygen-substituted lanthanum hydride. J. Am. Chem. Soc. 144, 1523–1527 (2022).
Google Scholar
Haile, S. M., Chisholm, C. R. I., Sasaki, K., Boysen, D. A. & Uda, T. Solid acid proton conductors: from laboratory curiosities to fuel cell electrolytes. Faraday Discuss. 134, 17–39 (2007).
Google Scholar
Kreuer, K. D. Proton-conducting oxides. Annu. Rev. Mater. Res. 33, 333–359 (2003).
Google Scholar
Fujii, S. et al. Emerging computational and machine learning methodologies for proton-conducting oxides: materials discovery and fundamental understanding. Sci. Technol. Adv. Mater. 25, 2416383 (2024).
Kreuer, K. D. Aspects of the formation and mobility of protonic charge carriers and the stability of perovskite-type oxides. Solid State Ion. 125, 285–302 (1999).
Google Scholar
Yamazaki, Y. et al. Proton trapping in yttrium-doped barium zirconate. Nat. Mater. 12, 647–651 (2013).
Google Scholar
Björketun, M. E., Sundell, P. G. & Wahnström, G. Structure and thermodynamic stability of hydrogen interstitials in BaZrO3 perovskite oxide from density functional calculations. Faraday Discuss. 134, 247–265 (2007).
Yamazaki, Y. et al. Oxygen affinity: the missing link enabling prediction of proton conductivities in doped barium zirconates. Chem. Mater. 32, 7292–7300 (2020).
Google Scholar
Hoshino, K. et al. Probing local environments of oxygen vacancies responsible for hydration in Sc-doped barium zirconates at elevated temperatures: in situ X-ray absorption spectroscopy, thermogravimetry, and active learning ab initio replica exchange Monte Carlo simulations. Chem. Mater. 35, 2289–2301 (2023).
Google Scholar
Iguchi, F., Tsurui, T., Sata, N., Nagao, Y. & Yugami, H. The relationship between chemical composition distributions and specific grain boundary conductivity in Y-doped BaZrO3 proton conductors. Solid State Ion. 180, 563–568 (2009).
Google Scholar
Imashuku, S., Uda, T., Nose, Y. & Awakura, Y. To Journal of Phase Equilibria and Diffusion phase relationship of the BaO-ZrO2-YO1.5 system at 1500 and 1600 °C. J. Phase Equilibria Diffus. 31, 348–356 (2010).
Google Scholar
Fabbri, E., Pergolesi, D., Licoccia, S. & Traversa, E. Does the increase in Y-dopant concentration improve the proton conductivity of BaZr1−xYxO3−δ fuel cell electrolytes? Solid State Ion. 181, 1043–1051 (2010).
Google Scholar
Toyoura, K., Meng, G., Han, D. & Uda, T. Preferential proton conduction along a three-dimensional dopant network in yttrium-doped barium zirconate: a first-principles study. J. Mater. Chem. A 6, 22721–22730 (2018).
Google Scholar
Imashuku, S., Uda, T., Ichitsubo, T., Matsubara, E. & Awakura, Y. A pseudoternary phase diagram of the BaO–ZrO2–ScO1.5 system at 1600 °C and solubility of scandia into barium zirconate. J. Phase Equilibria Diffus. 28, 517–522 (2007).
Google Scholar
Koichi, E. Ceramic materials containing rare earth oxides for solid oxide fuel cell. J. Alloy. Compd. 250, 486–491 (1997).
Draber, F. M. et al. Nanoscale percolation in doped BaZrO3 for high proton mobility. Nat. Mater. 19, 338–346 (2020).
Google Scholar
Saito, K. & Yashima, M. High proton conductivity within the ‘Norby gap’ by stabilizing a perovskite with disordered intrinsic oxygen vacancies. Nat. Commun. 14, 7466 (2023).
Steele, B. C. H. & Heinzel, A. Materials for fuel-cell technologies. Nature 414, 345–352 (2001).
Google Scholar
Li, L. & Nino, J. C. Proton-conducting barium stannates: doping strategies and transport properties. Int. J. Hydrog. Energy 38, 1598–1606 (2013).
Google Scholar
Kinyanjui, F. G. et al. Crystal structure and proton conductivity of BaSn0.6Sc0.4O3–δ: insights from neutron powder diffraction and solid-state NMR spectroscopy. J. Mater. Chem. A 4, 5088–5101 (2016).
Google Scholar
Kreuer, K. D., Münch, W., Fuchs, A., Klock, U. & Maier, J. Proton conducting alkaline earth zirconates and titanates for high drain electrochemical applications. Solid State Ion. 145, 295–306 (2001).
Google Scholar
Hyodo, J., Tsujikawa, K., Shiga, M., Okuyama, Y. & Yamazaki, Y. Accelerated discovery of proton-conducting perovskite oxide by capturing physicochemical fundamentals of hydration. ACS Energy Lett. 6, 2985–2992 (2021).
Google Scholar
Nowick, A. S. & Vaysleyb, A. V. Isotope effect and proton hopping in high-temperature protonic conductors. Solid State Ion. 97, 17–26 (1997).
Google Scholar
Yamada, S., Kanayama, K. & Toyoura, K. Nuclear quantum effects on proton diffusivity in perovskite oxides. Phys. Rev. B 111, 064117 (2025).
Google Scholar
Roy, T., Sahani, S., Madhu, D. & Chandra Sharma, Y. A clean approach of biodiesel production from waste cooking oil by using single phase BaSnO3 as solid base catalyst: mechanism, kinetics & E-study. J. Clean. Prod. 265, 121440 (2020).
Google Scholar
Haile, S. M., Staneff, G. & Ryu, K. H. Non-stoichiometry, grain boundary transport and chemical stability of proton conducting perovskites. J. Mater. Sci. 36, 1149–1160 (2001).
Google Scholar
Omata, T., Fuke, T., Otsuka, Y. & Matsuo, S. Hydration behavior of Ba2Sc2O5 with an oxygen-deficient perovskite structure. Solid State Ion. 177, 2447–2451 (2006).
Google Scholar
Cervera, R. B. et al. Perovskite-structured BaScO2(OH) as a novel proton conductor: heavily hydrated phase obtained via low-temperature synthesis. Chem. Mater. 25, 1483–1489 (2013).
Google Scholar
Saito, K., Umeda, K., Fujii, K., Mori, K. & Yashima, M. High proton conduction by full hydration in highly oxygen deficient perovskite. J. Mater. Chem. A 12, 13310–13319 (2024).
Google Scholar
Yamazaki, Y., Yang, C.-K. & Haile, S. M. Unraveling the defect chemistry and proton uptake of yttrium-doped barium zirconate. Scr. Mater. 65, 102–107 (2011).
Google Scholar
Han, D., Hatada, N. & Uda, T. Chemical expansion of yttrium‐doped barium zirconate and correlation with proton concentration and conductivity. J. Am. Ceram. Soc. 99, 3745–3753 (2016).
Google Scholar
Knight, K. S. & Bonanos, N. The crystal structures of some doped and undoped alkaline earth cerate perovskites. Mater. Res. Bull. 30, 347–356 (1995).
Google Scholar
Zheng, M. & Bo, Z. Proton conductivity in Yb-doped strontium cerates. Solid State Ion. 80, 59–65 (1995).
Google Scholar
Loureiro, F. J. A., Shakel, Z., Graça, V. C. D., Holz, L. I. V. & Fagg, D. P. Benchmarking the yttrium content in the low temperature/low humidity electrical properties of yttrium-doped barium cerate. Ceram. Int. 49, 34303–34308 (2023).
Google Scholar
Takeuchi, K. et al. The crystal structures and phase transitions in Y-doped BaCeO3: their dependence on Y concentration and hydrogen doping. Solid State Ion. 138, 63–77 (2000).
Google Scholar
Clark, D. et al. Anomalous low-temperature proton conductivity enhancement in a novel protonic nanocomposite. Phys. Chem. Chem. Phys. 16, 5076–5080 (2014).
Google Scholar
Nomura, K. et al. Phase transitions, thermal expansions, chemical expansions, and CO2 resistances of Ba(Ce0.8–xZrxY0.1Yb0.1)O3–δ (x = 0.1, 0.4) perovskite-type proton conductors. J. Electrochem. Soc. 169, 024516 (2022).
Google Scholar
Sone, Y., Ekdunge, P. & Simonsson, D. Proton conductivity of Nafion 117 as measured by a four-electrode AC impedance method. J. Electrochem. Soc. 143, 1254–1259 (1996).
Google Scholar
Nguyen, N. T. Q. & Yoon, H. H. Preparation and evaluation of BaZr0.1Ce0.7Y0.1Yb0.1O3−δ (BZCYYb) electrolyte and BZCYYb-based solid oxide fuel cells. J. Power Sources 231, 213–218 (2013).
Google Scholar
Mori, M. et al. Cubic-stabilized zirconia and alumina composites as electrolytes in planar type solid oxide fuel cells. Solid State Ion. 15, 157–164 (1994).
Jain, A. et al. Commentary: The Materials Project: a materials genome approach to accelerating materials innovation. APL Mater. 1, 011002 (2013).
Kasamatsu, S., Motoyama, Y., Yoshimi, K. & Aoyama, T. Configuration sampling in multi-component multi-sublattice systems enabled by ab Initio Configuration Sampling Toolkit (abICS). Sci. Technol. Adv. Mater. Methods 3, 2284128 (2023).
Kasamatsu, S. et al. Facilitating ab initio configurational sampling of multicomponent solids using an on-lattice neural network model and active learning. J. Chem. Phys. 157, 104114 (2022).
Google Scholar
Thompson, A. P. et al. LAMMPS – a flexible simulation tool for particle-based materials modeling at the atomic, meso, and continuum scales. Comput. Phys. Commun. 271, 108171 (2022).
Google Scholar
Musaelian, A. et al. Learning local equivariant representations for large-scale atomistic dynamics. Nat. Commun. 14, 579 (2023).
Google Scholar
Henkelman, G., Uberuaga, B. P. & Jónsson, H. A climbing image nudged elastic band method for finding saddle points and minimum energy paths. J. Chem. Phys. 113, 9901–9904 (2000).
Google Scholar
Fujii, S., Shimizu, Y., Hyodo, J., Kuwabara, A. & Yamazaki, Y. Discovery of unconventional proton‐conducting inorganic solids via defect‐chemistry‐trained, interpretable machine learning. Adv. Energy Mater. 13, 2301892 (2023).
Google Scholar