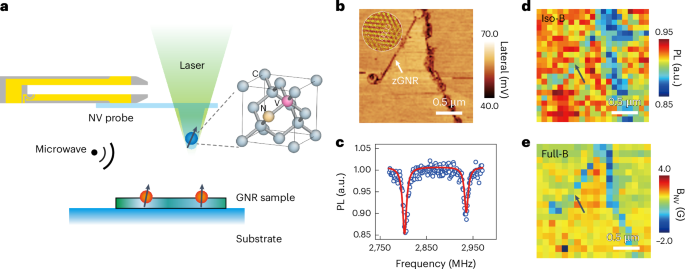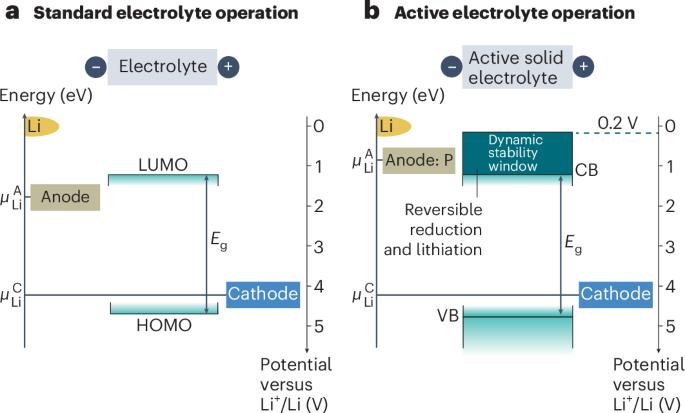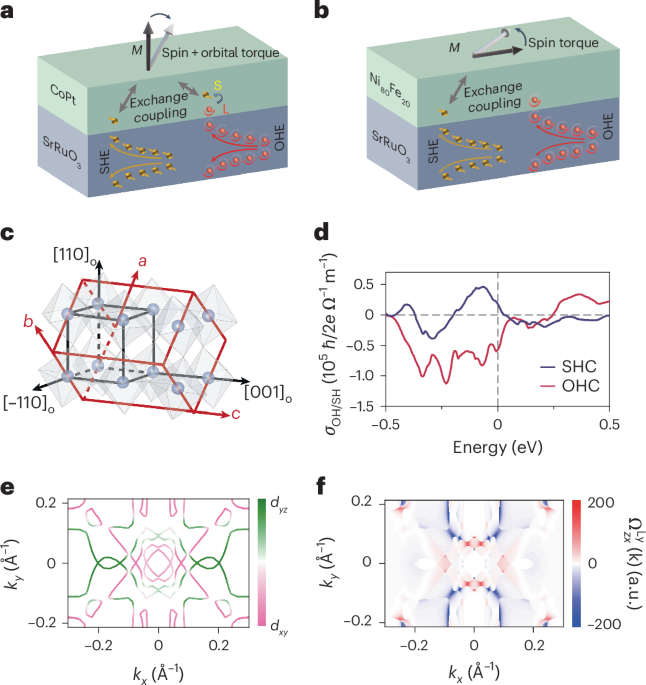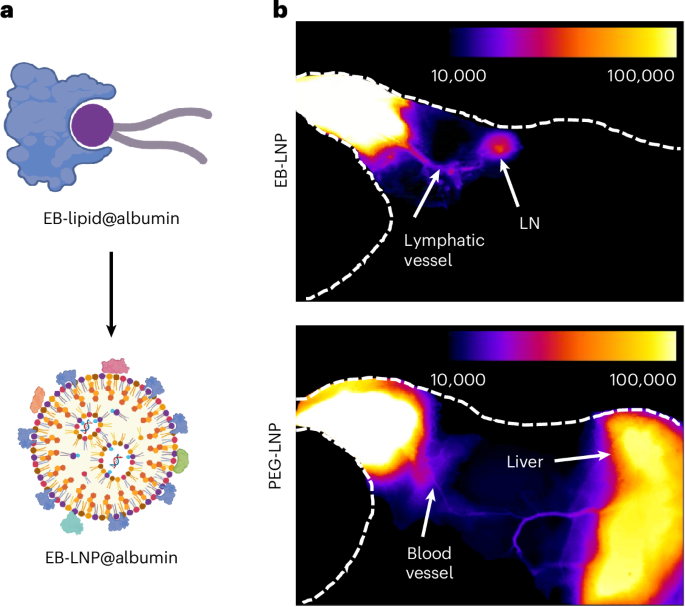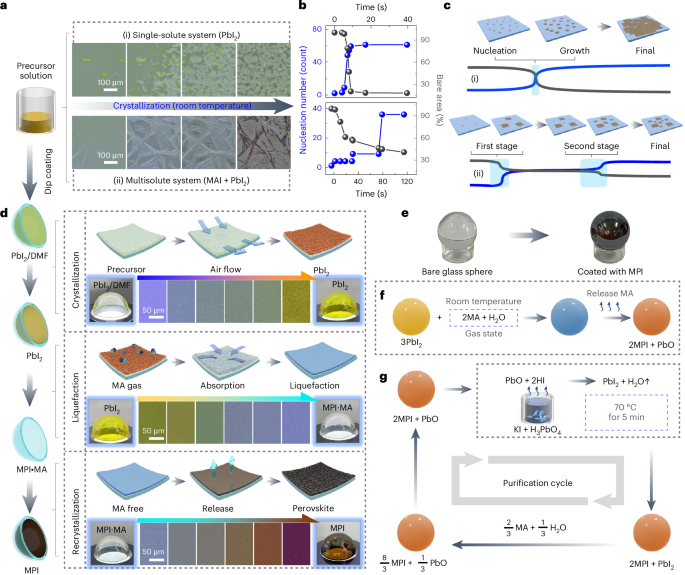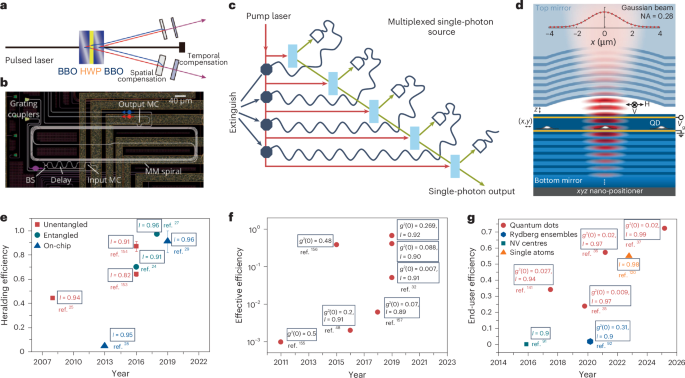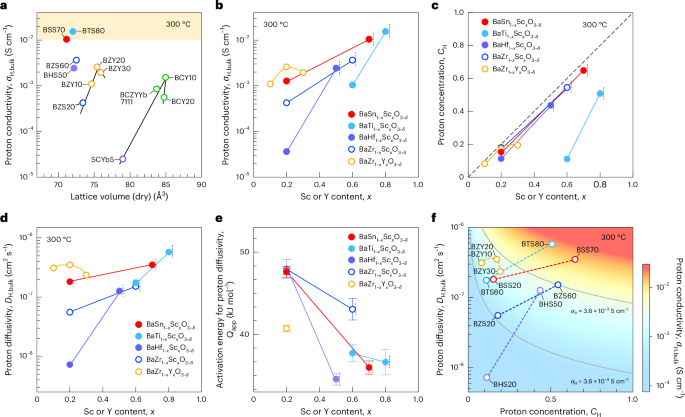
Pardi, N. et al. mRNA vaccines—a new era in vaccinology. Nat. Rev. Drug Discov. 17, 261–279 (2018).
Google Scholar
Hou, X. et al. Lipid nanoparticles for mRNA delivery. Nat. Rev. Mater. 6, 1078–1094 (2021).
Google Scholar
Pardi, N. & Krammer, F. mRNA vaccines for infectious diseases—advances, challenges and opportunities. Nat. Rev. Drug. Discov. 23, 838–861 (2024).
Google Scholar
Ju, Y. et al. Impact of anti-PEG antibodies induced by SARS-CoV-2 mRNA vaccines. Nat. Rev. Immunol. 23, 135–136 (2023).
Google Scholar
Chen, J. et al. Current developments and challenges of mRNA vaccines. Annu. Rev. Biomed. Eng. 24, 85–109 (2022).
Google Scholar
Weber, J. S. et al. Individualised neoantigen therapy mRNA-4157 (V940) plus pembrolizumab versus pembrolizumab monotherapy in resected melanoma (KEYNOTE-942): a randomised, phase 2b study. Lancet 403, 632–644 (2024).
Google Scholar
Ndeupen, S. et al. The mRNA-LNP platform’s lipid nanoparticle component used in preclinical vaccine studies is highly inflammatory. iScience 24, 103479 (2021).
Google Scholar
Pateev, I. et al. Biodistribution of RNA vaccines and of their products: evidence from human and animal studies. Biomedicines 12, 59 (2023).
Bahl, K. et al. Preclinical and clinical demonstration of immunogenicity by mRNA vaccines against H10N8 and H7N9 influenza viruses. Mol. Ther. 25, 1316–1327 (2017).
Google Scholar
Ye, Z. et al. Monovalent SARS-COV-2 mRNA vaccine using optimal UTRs and LNPs is highly immunogenic and broadly protective against Omicron variants. Proc. Natl Acad. Sci. USA 120, e2311752120 (2023).
Google Scholar
Shi, Y. et al. Structural and biochemical characteristics of mRNA nanoparticles determine anti-SARS-CoV-2 humoral and cellular immune responses. Sci. Adv. 8, eabo1827 (2022).
Google Scholar
Chen, J. et al. Lipid nanoparticle-mediated lymph node-targeting delivery of mRNA cancer vaccine elicits robust CD8+ T cell response. Proc. Natl Acad. Sci. USA 119, e2207841119 (2022).
Google Scholar
Kitagawa, H. et al. Adverse reactions to the BNT162b2 and mRNA-1273 mRNA COVID-19 vaccines in Japan. J. Infect. Chemother. 28, 576–581 (2022).
Google Scholar
Schinas, G. et al. Immune-mediated liver injury following COVID-19 vaccination. World J. Virol. 12, 100–108 (2023).
Efe, C. et al. Liver injury after SARS-CoV-2 vaccination: features of immune-mediated hepatitis, role of corticosteroid therapy and outcome. Hepatology 76, 1576–1586 (2022).
Google Scholar
Boettler, T. et al. SARS-CoV-2 vaccination can elicit a CD8 T-cell dominant hepatitis. J. Hepatol. 77, 653–659 (2022).
Google Scholar
Zin Tun, G. S. et al. Immune-mediated hepatitis with the Moderna vaccine, no longer a coincidence but confirmed. J. Hepatol. 76, 738–753 (2022).
Jeong, M. et al. Lipid nanoparticles (LNPs) for in vivo RNA delivery and their breakthrough technology for future applications. Adv. Drug Deliv. Rev. 200, 114990 (2023).
Google Scholar
Liu, M. et al. Lymph-targeted high-density lipoprotein-mimetic nanovaccine for multi-antigenic personalized cancer immunotherapy. Sci. Adv. 10, eadk2444 (2024).
Google Scholar
Sasaki, K. et al. mRNA-loaded lipid nanoparticles targeting dendritic cells for cancer immunotherapy. Pharmaceutics 14, 1572 (2022).
Google Scholar
Cheng, Q. et al. Selective organ targeting (SORT) nanoparticles for tissue-specific mRNA delivery and CRISPR–Cas gene editing. Nat. Nanotechnol. 15, 313–320 (2020).
Google Scholar
Liu, S. et al. Membrane-destabilizing ionizable phospholipids for organ-selective mRNA delivery and CRISPR–Cas gene editing. Nat. Mater. 20, 701–710 (2021).
Google Scholar
Yu, X. et al. Poly(ethyl ethylene phosphate): overcoming the ‘polyethylene glycol dilemma’ for cancer immunotherapy and mRNA vaccination. ACS Nano 17, 23814–23828 (2023).
Google Scholar
Hald Albertsen, C. et al. The role of lipid components in lipid nanoparticles for vaccines and gene therapy. Adv. Drug Deliv. Rev. 188, 114416 (2022).
Google Scholar
Tenchov, R. et al. PEGylated lipid nanoparticle formulations: immunological safety and efficiency perspective. Bioconjug. Chem. 34, 941–960 (2023).
Google Scholar
Dilliard, S. A. et al. On the mechanism of tissue-specific mRNA delivery by selective organ targeting nanoparticles. Proc. Natl Acad. Sci. USA 118, e210925611 (2021).
Elzoghby, A. O. et al. Albumin-based nanoparticles as potential controlled release drug delivery systems. J. Control. Release 157, 168–182 (2012).
Google Scholar
Abdallah, M. et al. Lymphatic targeting by albumin-hitchhiking: applications and optimisation. J. Control. Release 327, 117–128 (2020).
Google Scholar
Famta, P. et al. Albumin-hitchhiking: fostering the pharmacokinetics and anticancer therapeutics. J. Control. Release 353, 166–185 (2022).
Qi, S. et al. The bright future of nanotechnology in lymphatic system imaging and imaging-guided surgery. J. Nanobiotechnol. 20, 24 (2022).
Zhu, G. et al. Albumin/vaccine nanocomplexes that assemble in vivo for combination cancer immunotherapy. Nat. Commun. 8, 1954 (2017).
Um, W. et al. A comparative study on albumin-binding molecules for targeted tumor delivery through covalent and noncovalent approach. Bioconjug. Chem. 30, 3107–3118 (2019).
Google Scholar
Liu, H. et al. Structure-based programming of lymph-node targeting in molecular vaccines. Nature 507, 519–522 (2014).
Google Scholar
Song, Y. et al. Albumin nanoparticle containing a PI3Kγ inhibitor and paclitaxel in combination with α-PD1 induces tumor remission of breast cancer in mice. Sci. Transl. Med. 14, eabl3649 (2022).
Google Scholar
Lin, T. et al. Blood–brain-barrier-penetrating albumin nanoparticles for biomimetic drug delivery via albumin-binding protein pathways for antiglioma therapy. ACS Nano 10, 9999–10012 (2016).
Google Scholar
Liu, Z. & Chen, X. Simple bioconjugate chemistry serves great clinical advances: albumin as a versatile platform for diagnosis and precision therapy. Chem. Soc. Rev. 45, 1432–1456 (2016).
Google Scholar
Sahin, U. et al. BNT162b2 vaccine induces neutralizing antibodies and poly-specific T cells in humans. Nature 595, 572–577 (2021).
Google Scholar
Liu, Y. et al. Intrapleural nano-immunotherapy promotes innate and adaptive immune responses to enhance anti-PD-L1 therapy for malignant pleural effusion. Nat. Nanotechnol. 17, 206–216 (2022).
Google Scholar
Bern, M. et al. The role of albumin receptors in regulation of albumin homeostasis: implications for drug delivery. J. Control. Release 211, 144–162 (2015).
Google Scholar
Ishima, Y. et al. The new delivery strategy of albumin carrier utilizing the interaction with albumin receptors. Chem. Pharm. Bull. 70, 330–333 (2022).
Google Scholar
Wan, Y. et al. Stable organic photosensitizer nanoparticles with absorption peak beyond 800 nanometers and high reactive oxygen species yield for multimodality phototheranostics. ACS Nano 14, 9917–9928 (2020).
Google Scholar
Sindhwani, S. et al. The entry of nanoparticles into solid tumours. Nat. Mater. 19, 566–575 (2020).
Google Scholar
Nguyen, L. N. M. et al. The exit of nanoparticles from solid tumours. Nat. Mater. 22, 1261–1272 (2023).
Google Scholar
Embgenbroich, M. & Burgdorf, S. Current concepts of antigen cross-presentation. Front. Immunol. 9, 1643 (2018).
Oğuz, F. & Atmaca, H. mRNA as a therapeutics: understanding mRNA vaccines. Adv. Pharm. Bull. 12, 274–282 (2022).
Szymonowicz, K. A. & Chen, J. Biological and clinical aspects of HPV-related cancers. Cancer Biol. Med. 17, 864 (2020).
Google Scholar
Mirabello, L. et al. HPV16 E7 genetic conservation is critical to carcinogenesis. Cell 170, 1164–1174 (2017).
Google Scholar
Feshchenko, E. et al. Pandemic influenza vaccine: characterization of A/California/07/2009 (H1N1) recombinant hemagglutinin protein and insights into H1N1 antigen stability. BMC Biotechnol. 12, 77 (2012).
Google Scholar
Cyster, J. G. & Allen, C. D. C. B cell responses: cell interaction dynamics and decisions. Cell 177, 524–540 (2019).
Google Scholar
De Silva, N. S. & Klein, U. Dynamics of B cells in germinal centres. Nat. Rev. Immunol. 15, 137–148 (2015).
Mittenbühler, M. J. et al. Isolation of extracellular fluids reveals novel secreted bioactive proteins from muscle and fat tissues. Cell Metab. 35, 535–549 (2023).
Renier, N. et al. iDISCO: a simple, rapid method to immunolabel large tissue samples for volume imaging. Cell 159, 896–910 (2014).
Google Scholar