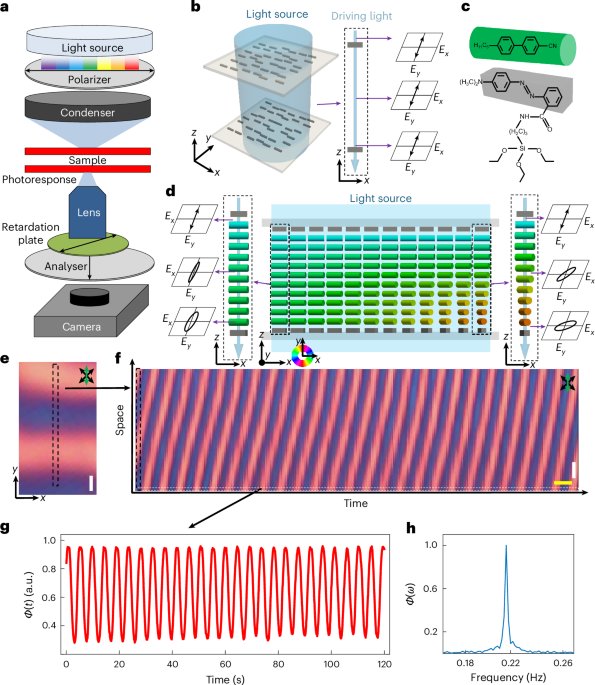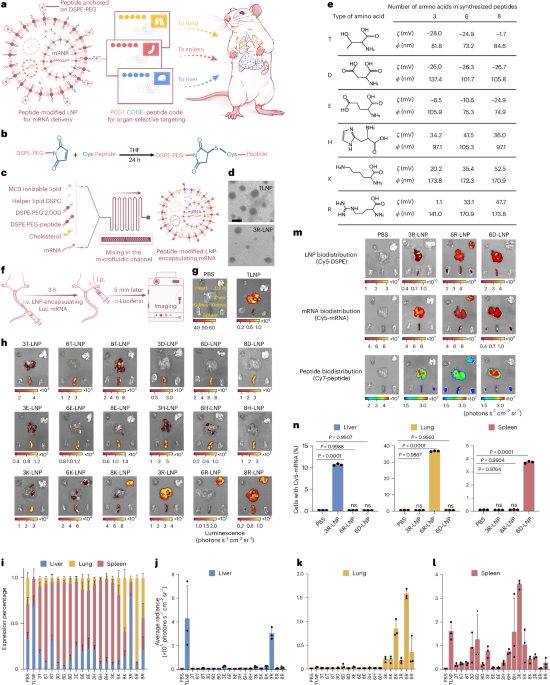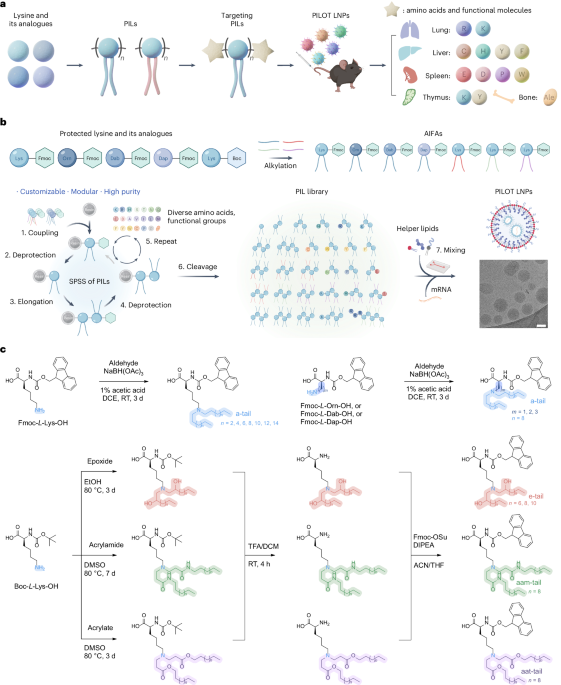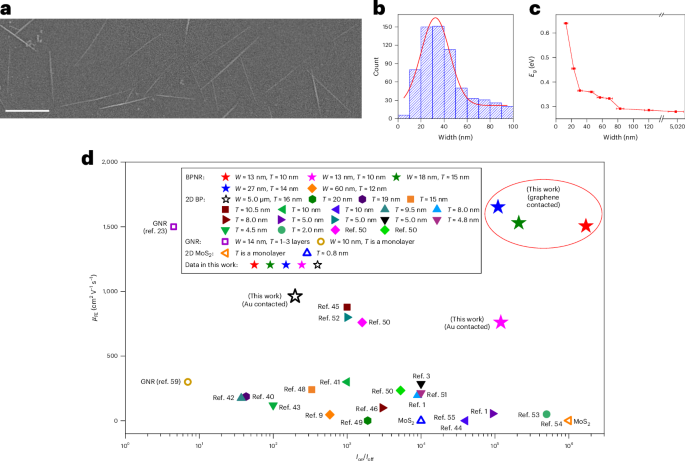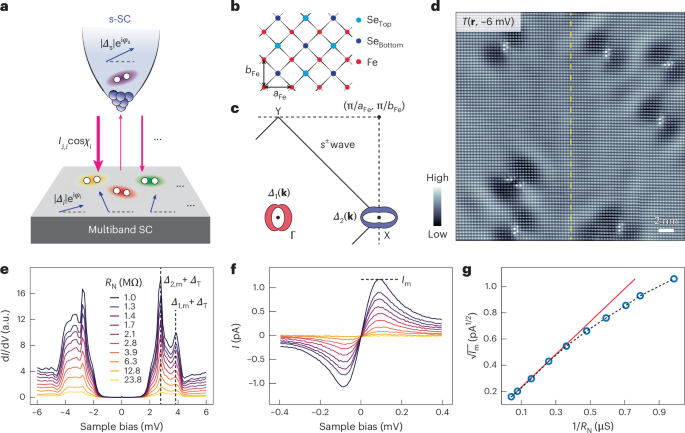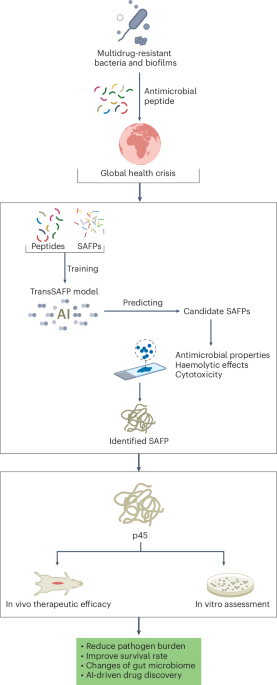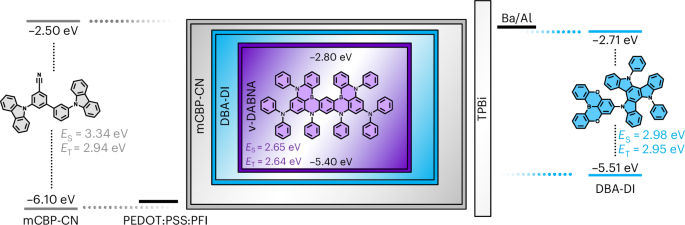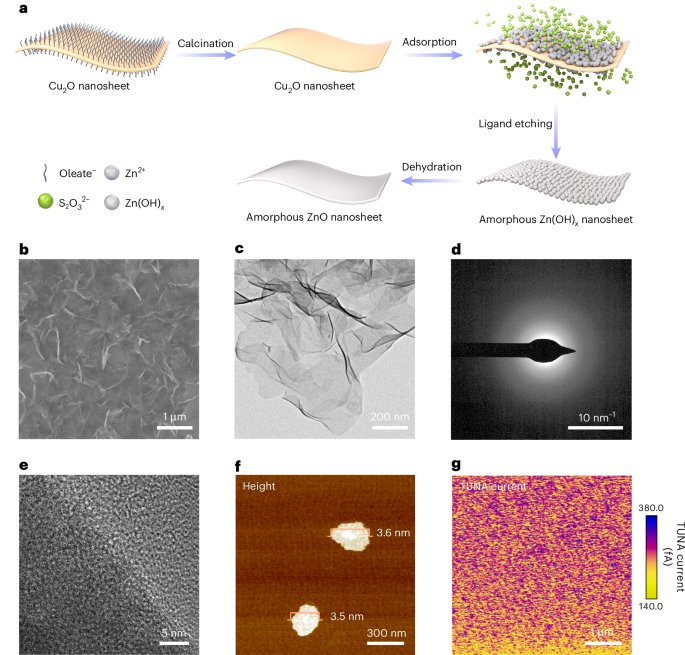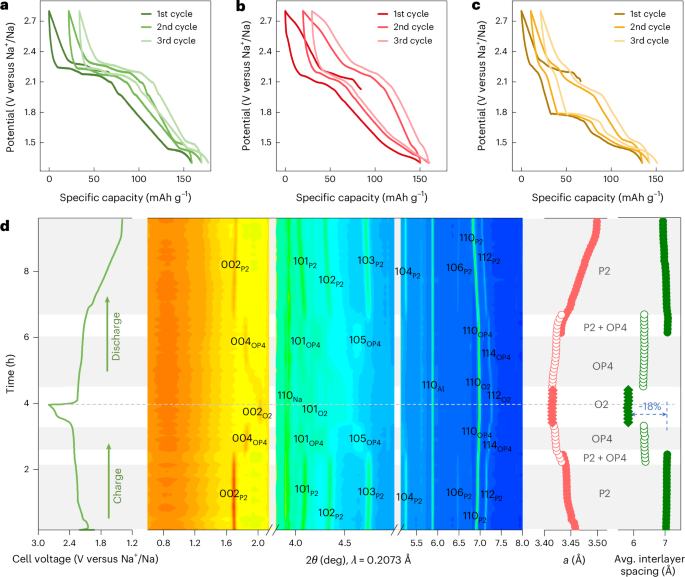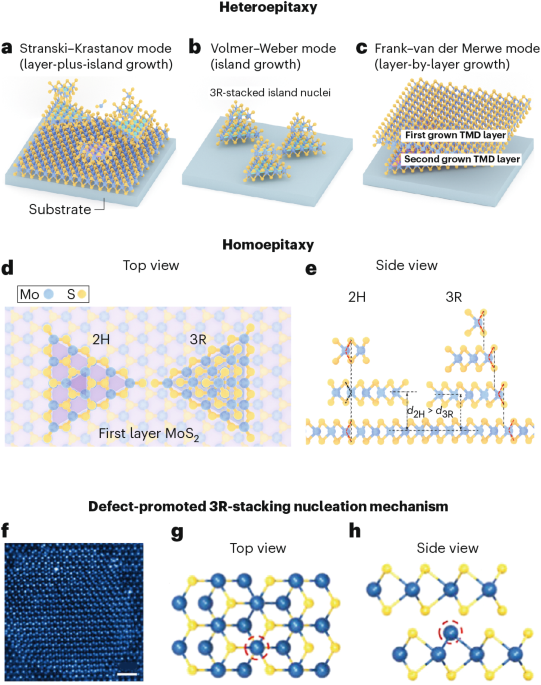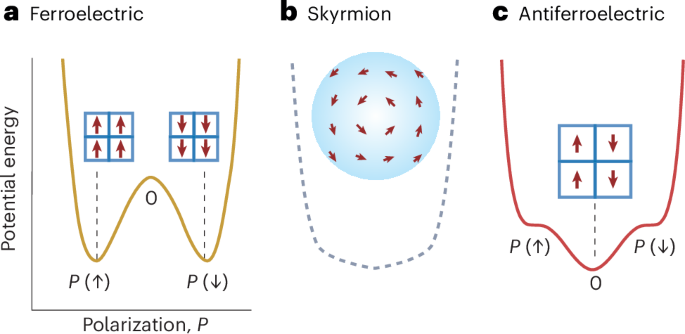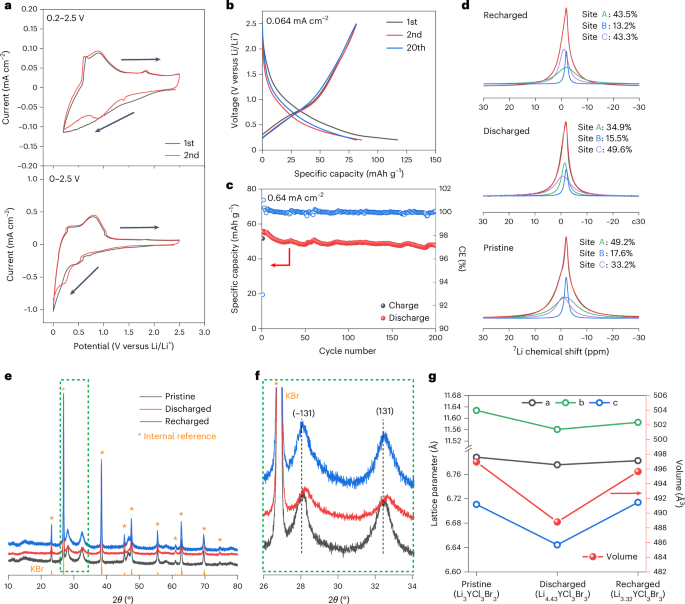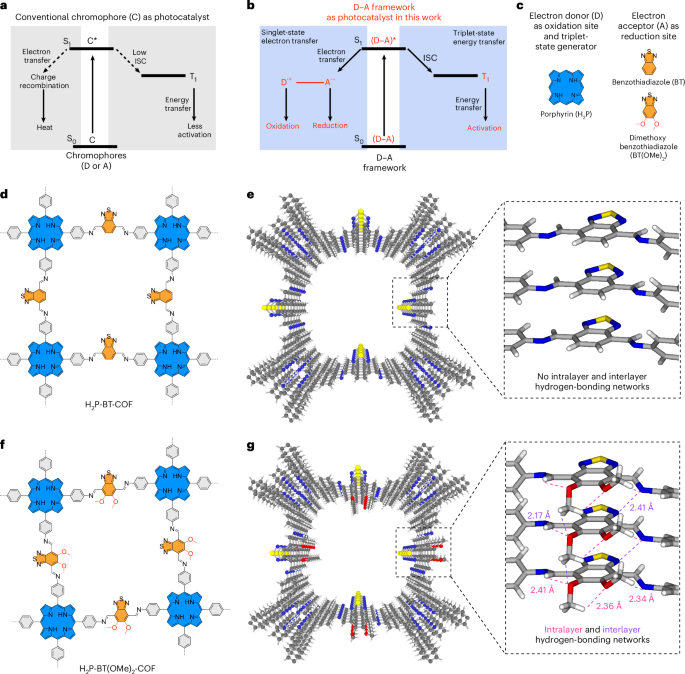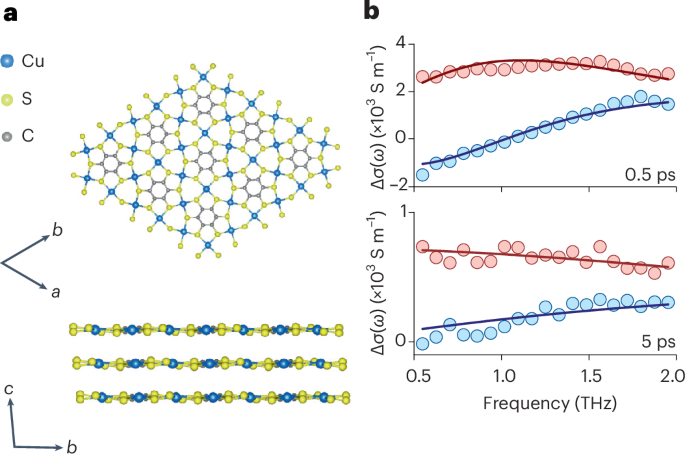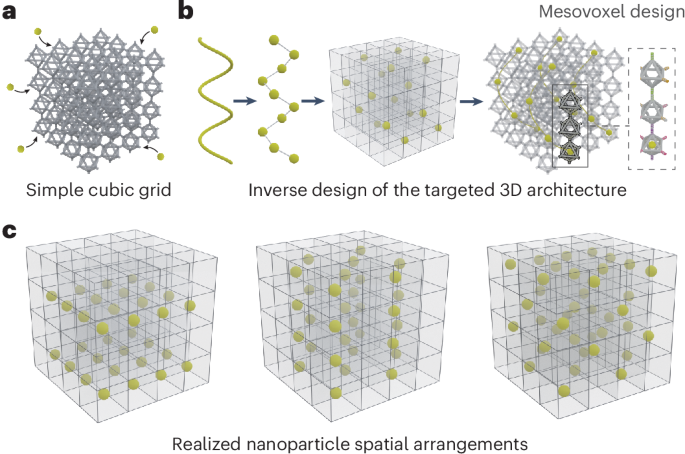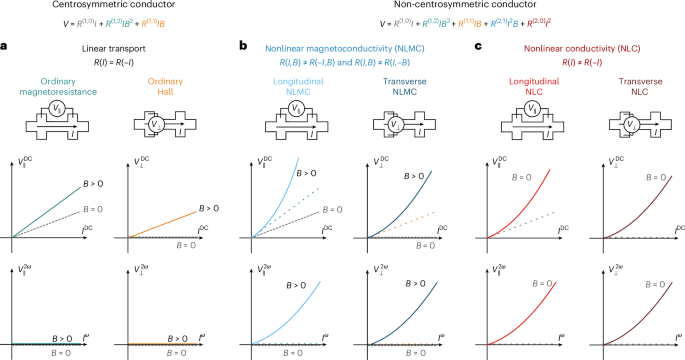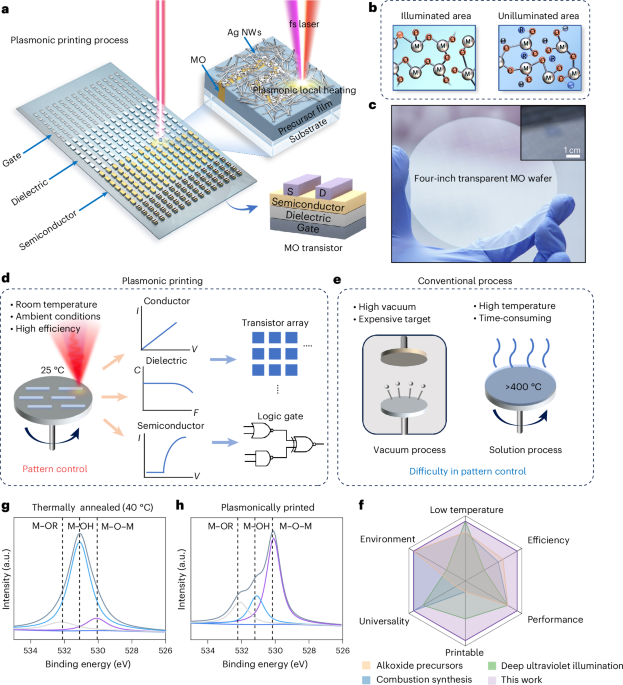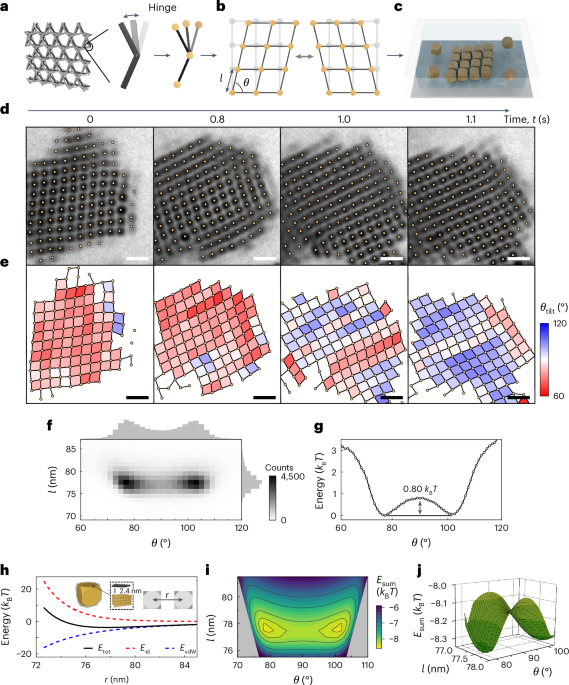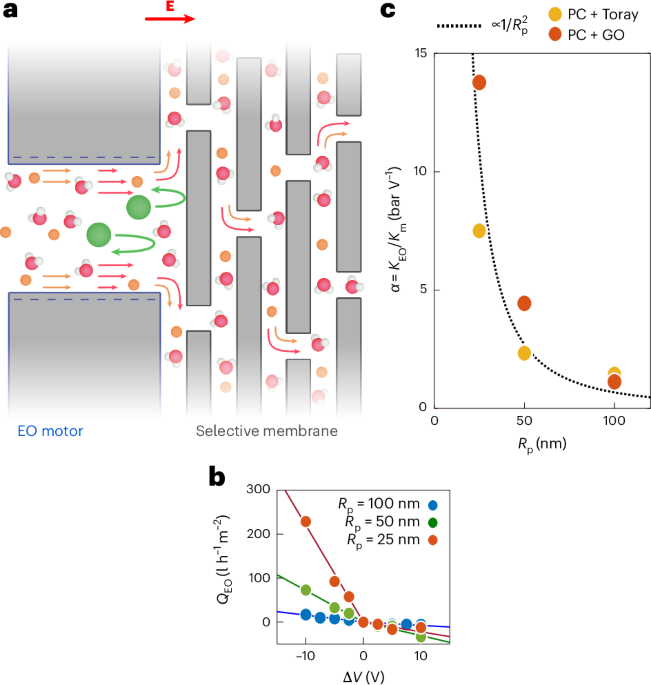
Goodenough, J. B. & Kim, Y. Challenges for rechargeable Li batteries. Chem. Mater. 22, 587–603 (2010).
Google Scholar
Mizushima, K., Jones, P., Wiseman, P. & Goodenough, J. B. LixCoO2 (0 < x < –1): a new cathode material for batteries of high energy density. Mater. Res. Bull. 15, 783–789 (1980).
Google Scholar
Padhi, A. K., Nanjundaswamy, K. S. & Goodenough, J. B. Phospho‐olivines as positive‐electrode materials for rechargeable lithium batteries. J. Electrochem. Soc. 144, 1188 (1997).
Google Scholar
Thackeray, M. M., David, W. I., Bruce, P. G. & Goodenough, J. B. Lithium insertion into manganese spinels. Mater. Res. Bull. 18, 461–472 (1983).
Google Scholar
Manthiram, A. A reflection on lithium-ion battery cathode chemistry. Nat. Commun. 11, 1550 (2020).
Google Scholar
Seo, D. H. et al. The structural and chemical origin of the oxygen redox activity in layered and cation-disordered Li-excess cathode materials. Nat. Chem. 8, 692–697 (2016).
Google Scholar
Lee, G.-H. et al. Reversible anionic redox activities in conventional LiNi1/3Co1/3Mn1/3O2 cathodes. Angew. Chem. Int. Ed. 59, 8681–8688 (2020).
Google Scholar
Lebens-Higgins, Z. W. et al. Revisiting the charge compensation mechanisms in LiNi0.8Co0.2−yAlyO2 systems. Mater. Horiz. 6, 2112–2123 (2019).
Google Scholar
Hua, W. et al. Structural insights into the formation and voltage degradation of lithium- and manganese-rich layered oxides. Nat. Commun. 10, 5365 (2019).
Google Scholar
Liu, T. et al. Origin of structural degradation in Li-rich layered oxide cathode. Nature 606, 305–312 (2022).
Google Scholar
Zeng, L. et al. Voltage decay of Li‐rich layered oxides: mechanism, modification strategies, and perspectives. Adv. Funct. Mater. 33, 2370151 (2023).
Zhang, K. et al. Sulfuration of Li‐rich Mn‐based cathode materials for multianionic redox and stabilized coordination environment. Adv. Mater. 34, 2109564 (2022).
Google Scholar
Li, B. et al. Decoupling the roles of Ni and Co in anionic redox activity of Li-rich NMC cathodes. Nat. Mater. 22, 1370–1379 (2023).
Google Scholar
Zhu, Z. et al. Gradient Li-rich oxide cathode particles immunized against oxygen release by a molten salt treatment. Nat. Energy 4, 1049–1058 (2019).
Google Scholar
House, R. A. et al. Superstructure control of first-cycle voltage hysteresis in oxygen-redox cathodes. Nature 577, 502–508 (2019).
Google Scholar
Song, J. et al. A high‐performance Li–Mn–O Li‐rich cathode material with rhombohedral symmetry via intralayer Li/Mn disordering. Adv. Mater. 32, 2000190 (2020).
Google Scholar
Huang, J. et al. Non-topotactic reactions enable high rate capability in Li-rich cathode materials. Nat. Energy 6, 706–714 (2021).
Google Scholar
Lee, J. et al. Unlocking the potential of cation-disordered oxides for rechargeable lithium batteries. Science 343, 519–522 (2014).
Google Scholar
Kim, J. & Manthiram, A. A manganese oxyiodide cathode for rechargeable lithium batteries. Nature 390, 265–267 (1997).
Google Scholar
Li, Y. et al. Origin of fast charging in hard carbon anodes. Nat. Energy 9, 134–142 (2024).
Google Scholar
Zhang, S. et al. A family of oxychloride amorphous solid electrolytes for long-cycling all-solid-state lithium batteries. Nat. Commun. 14, 3780 (2023).
Google Scholar
Heo, J. et al. Amorphous iron fluorosulfate as a high-capacity cathode utilizing combined intercalation and conversion reactions with unexpectedly high reversibility. Nat. Energy 8, 30–39 (2023).
Google Scholar
Kosova, N. V., Rezepova, D. O. & Slobodyuk, A. B. Effect of annealing temperature on the structure and electrochemistry of LiVO3. Electrochim. Acta 167, 75–83 (2015).
Google Scholar
Liu, Y., Zhou, X. & Guo, Y. Effects of fluorine doping on the electrochemical properties of LiV3O8 cathode material. Electrochim. Acta 54, 3184–3190 (2009).
Google Scholar
Chen, R. et al. Li+ intercalation in isostructural Li2VO3 and Li2VO2F with O2− and mixed O2−/F− anions. Phys. Chem. Chem. Phys. 17, 17288–17295 (2015).
Google Scholar
Ji, H. et al. Ultrahigh power and energy density in partially ordered lithium-ion cathode materials. Nat. Energy 5, 213–221 (2020).
Google Scholar
Li, S. et al. Facile synthesis of LiVO3 and its electrochemical behavior in rechargeable lithium batteries. J. Electroanal. Chem. 853, 113505 (2019).
Google Scholar
Juhás, P., Davis, T., Farrow, C. L. & Billinge, S. J. L. PDFgetX3: a rapid and highly automatable program for processing powder diffraction data into total scattering pair distribution functions. J. Appl. Crystallogr. 46, 560–566 (2013).
Terban, M. W. & Billinge, S. J. L. Structural analysis of molecular materials using the pair distribution function. Chem. Rev. 122, 1208–1272 (2021).
Google Scholar
Hu, E. et al. Evolution of redox couples in Li- and Mn-rich cathode materials and mitigation of voltage fade by reducing oxygen release. Nat. Energy 3, 690–698 (2018).
Google Scholar
Hoffmann, R. et al. From widely accepted concepts in coordination chemistry to inverted ligand fields. Chem. Rev. 116, 8173–8192 (2016).
Google Scholar
Sathiya, M. et al. Reversible anionic redox chemistry in high-capacity layered-oxide electrodes. Nat. Mater. 12, 827–835 (2013).
Google Scholar
Saubanère, M., McCalla, E., Tarascon, J.-M. & Doublet, M.-L. The intriguing question of anionic redox in high-energy density cathodes for Li-ion batteries. Energy Environ. Sci. 9, 984–991 (2016).
Zhang, Z. et al. Deciphering the critical effect of cathode-electrolyte interphase by revealing its dynamic evolution. J. Energy Chem. 81, 192–199 (2023).
Google Scholar
Zhang, J.-N. et al. Dynamic evolution of cathode electrolyte interphase (CEI) on high voltage LiCoO2 cathode and its interaction with Li anode. Energy Storage Mater. 14, 1–7 (2018).
Xu, J. et al. Elucidating anionic oxygen activity in lithium-rich layered oxides. Nat. Commun. 9, 947 (2018).
Google Scholar
Yang, W. & Devereaux, T. P. Anionic and cationic redox and interfaces in batteries: advances from soft X-ray absorption spectroscopy to resonant inelastic scattering. J. Power Sources 389, 188–197 (2018).
Google Scholar
Zhuo, Z. et al. Spectroscopic signature of oxidized oxygen states in peroxides. J. Phys. Chem. Lett. 9, 6378–6384 (2018).
Google Scholar
House, R. A. et al. Covalency does not suppress O2 formation in 4d and 5d Li-rich O-redox cathodes. Nat. Commun. 12, 2975 (2021).
Google Scholar
Li, X. et al. Direct visualization of the reversible O2−/O− redox process in Li-rich cathode materials. Adv. Mater. 30, 1705197 (2018).
Chen, H. & Islam, M. S. Lithium extraction mechanism in Li-rich Li2MnO3 involving oxygen hole formation and dimerization. Chem. Mater. 28, 6656–6663 (2016).
Google Scholar
Kawai, K. et al. Kinetic square scheme in oxygen-redox battery electrodes. Energy Environ. Sci. 15, 2591–2600 (2022).
Google Scholar
Li, B. et al. Electrolytic-anion-redox adsorption pseudocapacitance in nanosized lithium-free transition metal oxides as cathode materials for Li-ion batteries. Nano Energy 72, 104727 (2020).
Google Scholar
Ben Yahia, M., Vergnet, J., Saubanère, M. & Doublet, M.-L. Unified picture of anionic redox in Li/Na-ion batteries. Nat. Mater. 18, 496–502 (2019).
Google Scholar
Chen, Z., Li, J. & Zeng, X. C. Unraveling oxygen evolution in Li-rich oxides: a unified modeling of the intermediate peroxo/superoxo-like dimers. J. Am. Chem. Soc. 141, 10751–10759 (2019).
Google Scholar
McColl, K., Coles, S. W., Zarabadi-Poor, P., Morgan, B. J. & Islam, M. S. Phase segregation and nanoconfined fluid O2 in a lithium-rich oxide cathode. Nat. Mater. 23, 826–833 (2024).
Google Scholar
Suntivich, J. et al. Estimating hybridization of transition metal and oxygen states in perovskites from O K-edge X-ray absorption spectroscopy. J. Phys. Chem. C 118, 1856–1863 (2014).
Google Scholar
Schmitt, T. et al. High-resolution resonant inelastic X-ray scattering with soft X-rays at the ADRESS beamline of the Swiss light source: instrumental developments and scientific highlights. J. Electron. Spectrosc. Relat. Phenom. 188, 38–46 (2013).
Google Scholar
VandeVondele, J. et al. Quickstep: fast and accurate density functional calculations using a mixed Gaussian and plane waves approach. Comput. Phys. Commun. 167, 103–128 (2005).
Google Scholar
Kühne, T. D. et al. CP2K: an electronic structure and molecular dynamics software package—quickstep: efficient and accurate electronic structure calculations. J. Chem. Phys. 152, 194103 (2020).
Google Scholar
Krack, M. Pseudopotentials for H to Kr optimized for gradient-corrected exchange-correlation functionals. Theor. Chem. Acc. 114, 145–152 (2005).
Google Scholar
VandeVondele, J. & Hutter, J. Gaussian basis sets for accurate calculations on molecular systems in gas and condensed phases. J. Chem. Phys. 127, 114105 (2007).
Google Scholar
Jain, A. et al. Commentary: The Materials Project: a materials genome approach to accelerating materials innovation. APL Mater. 1, 011002 (2013).