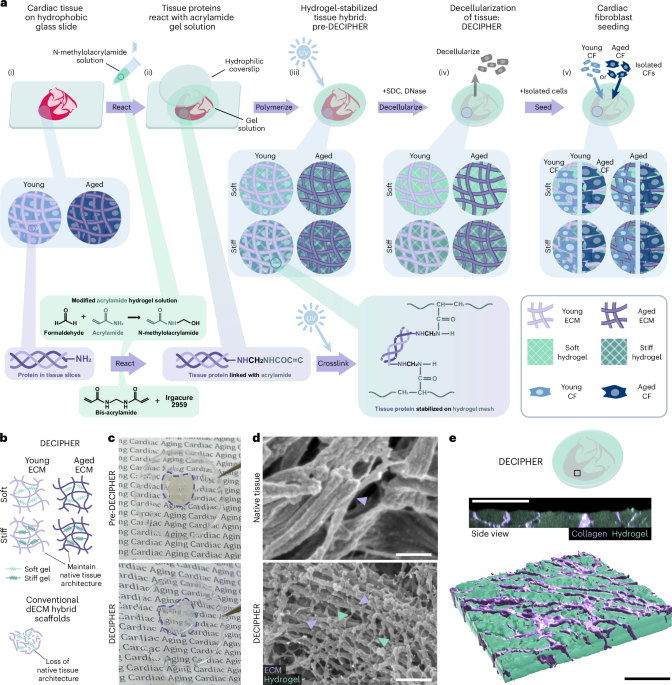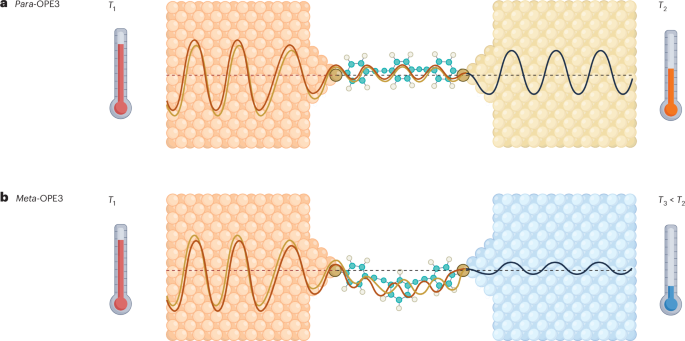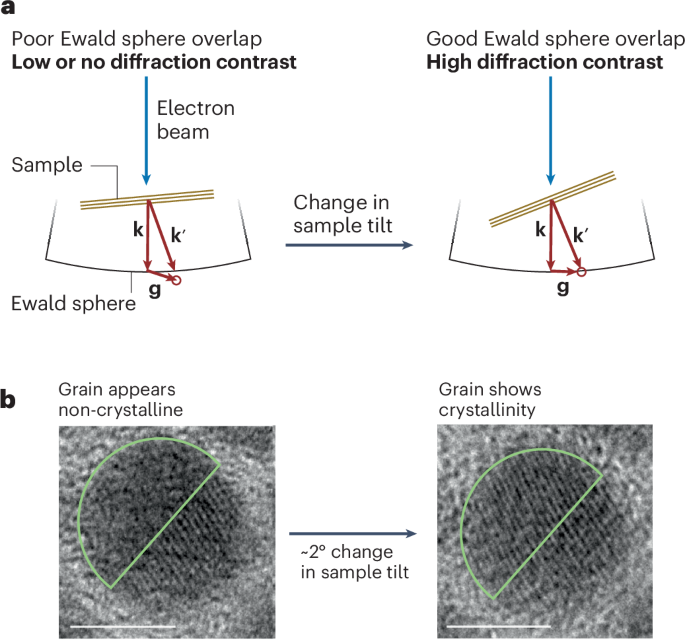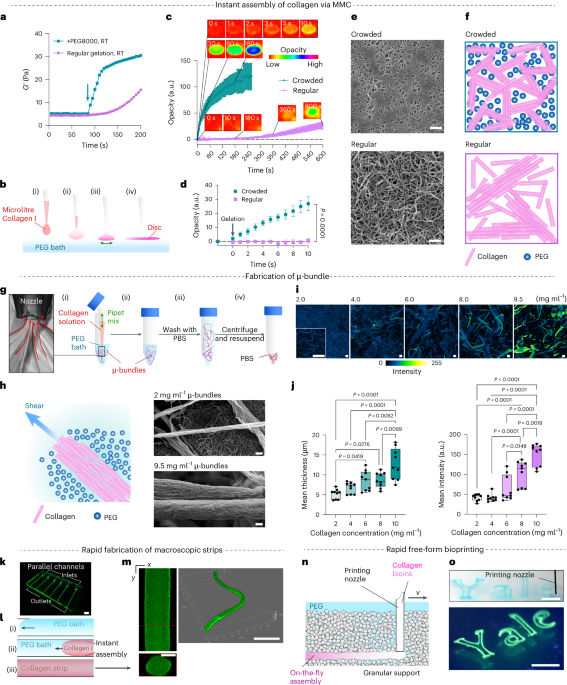
Wang, Q., Gao, Q., Al-Enizi, A. M., Nafady, A. & Ma, S. Recent advances in MOF-based photocatalysis: environmental remediation under visible light. Inorg. Chem. Front. 7, 300–339 (2020).
Google Scholar
Wang, H. et al. A review on heterogeneous photocatalysis for environmental remediation: from semiconductors to modification strategies. Chin. J. Catal. 43, 178–214 (2022).
Google Scholar
Banerjee, T., Podjaski, F., Kröger, J., Biswal, B. P. & Lotsch, B. V. Polymer photocatalysts for solar-to-chemical energy conversion. Nat. Rev. Mater. 6, 168–190 (2021).
Google Scholar
He, T. & Zhao, Y. Covalent organic frameworks for energy conversion in photocatalysis. Angew. Chem. Int. Ed. 62, e202303086 (2023).
Google Scholar
Douglas, J. J., Sevrin, M. J. & Stephenson, C. R. J. Visible light photocatalysis: applications and new disconnections in the synthesis of pharmaceutical agents. Org. Process Res. Dev. 20, 1134–1147 (2016).
Google Scholar
Twilton, J. et al. The merger of transition metal and photocatalysis. Nat. Rev. Chem. 1, 0052 (2017).
Google Scholar
Bellotti, P., Huang, H.-M., Faber, T. & Glorius, F. Photocatalytic late-stage C–H functionalization. Chem. Rev. 123, 4237–4352 (2023).
Google Scholar
Crisenza, G. E. M. & Melchiorre, P. Chemistry glows green with photoredox catalysis. Nat. Commun. 11, 803 (2020).
Google Scholar
De Kreijger, S., Glaser, F. & Troian-Gautier, L. From photons to reactions: key concepts in photoredox catalysis. Chem. Catal. 4, 101110 (2024).
Prier, C. K., Rankic, D. A. & MacMillan, D. W. C. Visible light photoredox catalysis with transition metal complexes: applications in organic synthesis. Chem. Rev. 113, 5322–5363 (2013).
Google Scholar
Yoon, T. P., Ischay, M. A. & Du, J. Visible light photocatalysis as a greener approach to photochemical synthesis. Nat. Chem. 2, 527–532 (2010).
Google Scholar
Chen, Y. & Jiang, D. Photocatalysis with covalent organic frameworks. Acc. Chem. Res. 57, 3182–3193 (2024).
Google Scholar
Mishra, B. et al. Covalent organic frameworks for photocatalysis. Adv. Mater. 50, 2413118 (2025).
Wang, H. et al. Covalent organic framework photocatalysts: structures and applications. Chem. Soc. Rev. 49, 4135–4165 (2020).
Google Scholar
Sun, K. et al. Energy-transfer-enabled photocatalytic transformations of aryl thianthrenium salts. Nat. Commun. 15, 9693 (2024).
Google Scholar
Strieth-Kalthoff, F. & Glorius, F. Triplet energy transfer photocatalysis: unlocking the next level. Chem 6, 1888–1903 (2020).
Google Scholar
Schmitz, M., Bertrams, M.-S., Sell, A. C., Glaser, F. & Kerzig, C. Efficient energy and electron transfer photocatalysis with a coulombic dyad. J. Am. Chem. Soc. 146, 25799–25812 (2024).
Google Scholar
Closs, G. L. & Miller, J. R. Intramolecular long-distance electron transfer in organic molecules. Science 240, 440–447 (1988).
Google Scholar
Ghogare, A. A. & Greer, A. Using singlet oxygen to synthesize natural products and drugs. Chem. Rev. 116, 9994–10034 (2016).
Google Scholar
Singh, N., Sen Gupta, R. & Bose, S. A comprehensive review on singlet oxygen generation in nanomaterials and conjugated polymers for photodynamic therapy in the treatment of cancer. Nanoscale 16, 3243–3268 (2024).
Google Scholar
Tian, Y. et al. Heavy-atom-free covalent organic frameworks for organic room-temperature phosphorescence via Förster and Dexter energy transfer mechanism. Small Methods 9, 2401083 (2024).
Liu, X. et al. Triazine–porphyrin-based hyperconjugated covalent organic framework for high-performance photocatalysis. J. Am. Chem. Soc. 144, 23396–23404 (2022).
Google Scholar
Li, P., Dong, X., Zhang, Y., Lang, X. & Wang, C. An azine-linked 2D porphyrinic covalent organic framework for red light photocatalytic oxidative coupling of amines. Mater. Today Chem. 25, 100953 (2022).
Google Scholar
Wu, C. et al. Porphyrin covalent organic framework for photocatalytic synthesis of tetrahydroquinolines. Chin. Chem. Lett. 33, 4559–4562 (2022).
Google Scholar
Li, J. et al. Rationally modulating thiophene- and porphyrin-based donor–acceptor type covalent organic framework for effective photocatalytic organic reactions. Appl. Organomet. Chem. 38, e7605 (2024).
Google Scholar
Xia, Y., Zhang, W., Yang, S., Wang, L. & Yu, G. Research progress in donor–acceptor type covalent organic frameworks. Adv. Mater. 35, 2301190 (2023).
Google Scholar
Jin, S. et al. Creation of superheterojunction polymers via direct polycondensation: segregated and bicontinuous donor–acceptor π-columnar arrays in covalent organic frameworks for long-lived charge separation. J. Am. Chem. Soc. 137, 7817–7827 (2015).
Google Scholar
Jin, S. et al. Charge dynamics in a donor–acceptor covalent organic framework with periodically ordered bicontinuous heterojunctions. Angew. Chem. Int. Ed. 52, 2017–2021 (2013).
Google Scholar
Chen, Y. et al. Hierarchical assembly of donor–acceptor covalent organic frameworks for photosynthesis of hydrogen peroxide from water and air. Nat. Synth. 3, 998–1010 (2024).
Google Scholar
Zhao, S. et al. Hydrophilicity gradient in covalent organic frameworks for membrane distillation. Nat. Mater. 20, 1551–1558 (2021).
Google Scholar
Fresch, E. & Collini, E. The role of H-bonds in the excited-state properties of multichromophoric systems: static and dynamic aspects. Molecules 28, 3553 (2023).
Google Scholar
Li, Y. Molecular design of photovoltaic materials for polymer solar cells: toward suitable electronic energy levels and broad absorption. Acc. Chem. Res. 45, 723–733 (2012).
Google Scholar
Pawley, G. S. Unit-cell refinement from powder diffraction scans. J. Appl. Crystallogr. 14, 357–361 (1981).
Google Scholar
Uribe-Romo, F. J. et al. A crystalline imine-linked 3-D porous covalent organic framework. J. Am. Chem. Soc. 131, 4570–4571 (2009).
Google Scholar
Shinde, D. B., Kandambeth, S., Pachfule, P., Kumar, R. R. & Banerjee, R. Bifunctional covalent organic frameworks with two dimensional organocatalytic micropores. Chem. Commun. 51, 310–313 (2015).
Google Scholar
Thommes, M. et al. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl. Chem. 87, 1051–1069 (2015).
Google Scholar
Zhou, T. et al. PEG-stabilized coaxial stacking of two-dimensional covalent organic frameworks for enhanced photocatalytic hydrogen evolution. Nat. Commun. 12, 3934 (2021).
Google Scholar
Chen, R. et al. Rational design of isostructural 2D porphyrin-based covalent organic frameworks for tunable photocatalytic hydrogen evolution. Nat. Commun. 12, 1354 (2021).
Google Scholar
Blätte, D., Ortmann, F. & Bein, T. Photons, excitons, and electrons in covalent organic frameworks. J. Am. Chem. Soc. 146, 32161–32205 (2024).
Google Scholar
Ghosh, S. et al. Identification of prime factors to maximize the photocatalytic hydrogen evolution of covalent organic frameworks. J. Am. Chem. Soc. 142, 9752–9762 (2020).
Google Scholar
Gordo-Lozano, M. et al. Boosting photoconductivity by increasing the structural complexity of multivariate covalent organic frameworks. Small 21, 2406211 (2025).
Google Scholar
Chen, L. et al. The non-covalent assembly of benzene-bridged metallosalphen dimers: photoconductive tapes with large carrier mobility and spatially distinctive conduction anisotropy. Chem. Commun. 7, 3119–3121 (2009).
Tajima, K. et al. Synthesis and electron-transporting properties of phenazine bisimides. J. Mater. Chem. C 13, 655–662 (2025).
Google Scholar
Feng, X. et al. An ambipolar conducting covalent organic framework with self-sorted and periodic electron donor–acceptor ordering. Adv. Mater. 24, 3026–3031 (2012).
Google Scholar
Luo, Z. et al. Manipulating p–π resonance through methoxy group engineering in covalent organic frameworks for an efficient photocatalytic hydrogen evolution. Angew. Chem. Int. Ed. 64, e202420217 (2025).
Google Scholar
Xiong, L. & Tang, J. Strategies and challenges on selectivity of photocatalytic oxidation of organic substances. Adv. Energy Mater. 11, 2003216 (2021).
Google Scholar
Wen, Y., Yan, J., Yang, B., Zhuang, Z. & Yu, Y. Reactive oxygen species on transition metal-based catalysts for sustainable environmental applications. J. Mater. Chem. A 10, 19184–19210 (2022).
Google Scholar
Zhou, Z., Song, J., Nie, L. & Chen, X. Reactive oxygen species generating systems meeting challenges of photodynamic cancer therapy. Chem. Soc. Rev. 45, 6597–6626 (2016).
Google Scholar
Yang, B., Chen, Y. & Shi, J. Reactive oxygen species (ROS)-based nanomedicine. Chem. Rev. 119, 4881–4985 (2019).
Google Scholar
Shinkarenko, N. V. & Aleskovskii, V. B. Singlet oxygen: methods of preparation and detection. Russ. Chem. Rev. 50, 220 (1981).
Partanen, S. B., Erickson, P. R., Latch, D. E., Moor, K. J. & McNeill, K. Dissolved organic matter singlet oxygen quantum yields: evaluation using time-resolved singlet oxygen phosphorescence. Environ. Sci. Technol. 54, 3316–3324 (2020).
Google Scholar
Entradas, T., Waldron, S. & Volk, M. The detection sensitivity of commonly used singlet oxygen probes in aqueous environments. J. Photochem. Photobiol. B 204, 111787 (2020).
Google Scholar
Nagai, A. et al. A squaraine-linked mesoporous covalent organic framework. Angew. Chem. Int. Ed. 52, 3770–3774 (2013).
Google Scholar
Sun, N. et al. Photoresponsive covalent organic frameworks with diarylethene switch for tunable singlet oxygen generation. Chem. Mater. 34, 1956–1964 (2022).
Google Scholar
Feng, X. et al. Two-dimensional artificial light-harvesting antennae with predesigned high-order structure and robust photosensitising activity. Sci. Rep. 6, 32944 (2016).
Google Scholar
Park, K. C., Cho, J. & Lee, C. Y. Porphyrin and pyrene-based conjugated microporous polymer for efficient sequestration of CO2 and iodine and photosensitization for singlet oxygen generation. RSC Adv. 6, 75478–75481 (2016).
Google Scholar
Peng, Y.-Z. et al. Charge transfer from donor to acceptor in conjugated microporous polymer for enhanced photosensitization. Angew. Chem. Int. Ed. 60, 22062–22069 (2021).
Google Scholar
Zhang, W. et al. Combining ruthenium(II) complexes with metal–organic frameworks to realize effective two-photon absorption for singlet oxygen generation. ACS Appl. Mater. Interfaces 8, 21465–21471 (2016).
Google Scholar
Xue, F. et al. Iridium complex loaded polypyrrole nanoparticles for NIR laser induced photothermal effect and generation of singlet oxygen. RSC Adv. 6, 15509–15512 (2016).
Google Scholar
Finkelstein, E., Rosen, G. M. & Rauckman, E. J. Spin trapping of superoxide and hydroxyl radical: practical aspects. Arch. Biochem. Biophys. 200, 1–16 (1980).
Google Scholar
Choudhury, L. H. & Parvin, T. Recent advances in the chemistry of imine-based multicomponent reactions (MCRs). Tetrahedron 67, 8213–8228 (2011).
Google Scholar
Kobayashi, S. & Ishitani, H. Catalytic enantioselective addition to imines. Chem. Rev. 99, 1069–1094 (1999).
Google Scholar
Salahuddin, Shaharyar, M. & Mazumder, A. Benzimidazoles: a biologically active compound. Arab. J. Chem. 10, S157–S173 (2017).
Google Scholar
Gaba, M., Singh, S. & Mohan, C. Benzimidazole: an emerging scaffold for analgesic and anti-inflammatory agents. Eur. J. Med. Chem. 76, 494–505 (2014).
Google Scholar
Bagdi, A. K. et al. Visible light promoted cross-dehydrogenative coupling: a decade update. Green. Chem. 22, 6632–6681 (2020).
Google Scholar
Tian, T., Li, Z. & Li, C.-J. Cross-dehydrogenative coupling: a sustainable reaction for C–C bond formations. Green. Chem. 23, 6789–6862 (2021).
Google Scholar
Shi, J.-L. et al. 2D sp2 carbon-conjugated porphyrin covalent organic framework for cooperative photocatalysis with TEMPO. Angew. Chem. Int. Ed. 59, 9088–9093 (2020).
Google Scholar
Johnson, J. A. et al. Porphyrin-metalation-mediated tuning of photoredox catalytic properties in metal–organic frameworks. ACS Catal. 5, 5283–5291 (2015).
Google Scholar
Battula, V. R. et al. Natural sunlight driven oxidative homocoupling of amines by a truxene-based conjugated microporous polymer. ACS Catal. 8, 6751–6759 (2018).
Google Scholar
Su, F. et al. Aerobic oxidative coupling of amines by carbon nitride photocatalysis with visible light. Angew. Chem. Int. Ed. 50, 657–660 (2011).
Google Scholar
Zhang, N. et al. Oxide defect engineering enables to couple solar energy into oxygen activation. J. Am. Chem. Soc. 138, 8928–8935 (2016).
Google Scholar
Luo, B. et al. Benzotrithiophene and triphenylamine based covalent organic frameworks as heterogeneous photocatalysts for benzimidazole synthesis. J. Catal. 402, 52–60 (2021).
Google Scholar
Han, S. et al. Bandgap engineering in benzotrithiophene-based conjugated microporous polymers: a strategy for screening metal-free heterogeneous photocatalysts. J. Mater. Chem. A 9, 3333–3340 (2021).
Google Scholar
Razavi, S. A. A. et al. Redox metal–organic framework for photocatalytic organic transformation: the role of tetrazine function in radical-anion pathway. Inorg. Chem. 61, 19134–19143 (2022).
Google Scholar
Wang, C.-A., Han, Y.-F., Nie, K. & Li, Y.-W. Porous organic frameworks with mesopores and [Ru(bpy)3]2+ ligand built-in as a highly efficient visible-light heterogeneous photocatalyst. Mater. Chem. Front. 3, 1909–1917 (2019).
Google Scholar
Li, Z. et al. Visible-light-induced condensation cyclization to synthesize benzimidazoles using fluorescein as a photocatalyst. Green. Chem. 21, 3602–3605 (2019).
Google Scholar
Wang, C., Xie, Z., deKrafft, K. & Lin, W. Doping meta–organic frameworks for water oxidation, carbon dioxide reduction and organic photocatalysis. J. Am. Chem. Soc. 133, 13445–13454 (2011).
Google Scholar
Zhi, Y. et al. Covalent organic frameworks as metal-free heterogeneous photocatalysts for organic transformations. J. Mater. Chem. A 5, 22933–22938 (2017).
Google Scholar
Che, Y. et al. Ultrasmall Ag nanoparticles on photoactive metal–organic framework boosting aerobic cross-dehydrogenative coupling under visible light. Appl. Surf. Sci. 634, 157699 (2023).
Google Scholar
Sharma, N., Chauhan, D. K., Saini, N. & Kailasam, K. Metal-free triazine-based polymeric network for solar-to-chemical conversion: an insight into the aza-Henry reaction. ACS Appl. Polym. Mater. 5, 4333–4341 (2023).
Google Scholar
Ma, W. et al. Phosphorescent bismoviologens for electrophosphorochromism and visible light-induced cross-dehydrogenative coupling. J. Am. Chem. Soc. 143, 1590–1597 (2021).
Google Scholar
Liu, W. et al. Difluoroborate-based conjugated organic polymer: a high-performance heterogeneous photocatalyst for oxidative coupling reactions. J. Mater. Sci. 54, 1205–1212 (2019).
Google Scholar
Wang, H. et al. α-Cyanation of aromatic tertiary amines using malononitrile as a low-toxic cyanide source under the catalysis of NiGa layered double oxide. Asian J. Org. Chem. 9, 1769–1773 (2020).
Google Scholar
Liu, R. et al. Linkage-engineered donor–acceptor covalent organic frameworks for optimal photosynthesis of hydrogen peroxide from water and air. Nat. Catal. 7, 195–206 (2024).
Oleg, V. et al. OLEX2: a complete structure solution refinement and analysis program. J. Appl. Cryst. 42, 339–341 (2009).
Sheldrick, G. M. SHELXT – Integrated space-group and crystal-structure determination. Acta Crystallogr. A Found. Adv. 71, 3–8 (2015).
Google Scholar
Sheldrick, G. M. Crystal structure refinement with SHELXL. Acta Crystallogr. C Struct. Chem. 71, 3–8 (2015).
Google Scholar
Thompson, P., Cox, D. E. & Hastings, J. B. Rietveld refinement of Debye–Scherrer synchrotron X-ray data from Al2O3. J. Appl. Crystallogr. 20, 79–83 (1987).
Google Scholar
Bérar, J.-F. & Baldinozzi, G. Modeling of line-shape asymmetry in powder diffraction. J. Appl. Crystallogr. 26, 128–129 (1993).
Elstner, M. et al. Self-consistent-charge density-functional tight-binding method for simulations of complex materials properties. Phys. Rev. B 58, 7260–7268 (1998).
Google Scholar
Aradi, B., Hourahine, B. & Frauenheim, T. DFTB+, a sparse matrix-based implementation of the DFTB method. J. Phys. Chem. A 111, 5678–5684 (2007).
Google Scholar
Hourahine, B. et al. DFTB+, a software package for efficient approximate density functional theory based atomistic simulations. J. Chem. Phys. 152, 124101 (2020).
Google Scholar
Clark, S. J. et al. First principles methods using CASTEP. Zeitschrift für Kristallographie – Crystalline Materials 220, 567–570 (2005).
Google Scholar
Perdew, J. P., Burke, K. & Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 77, 3865–3868 (1996).
Google Scholar
Steiner, T. The hydrogen bond in the solid state. Angew. Chem. Int. Ed. 41, 48–76 (2002).
Google Scholar
Qin, C. et al. Dual donor–acceptor covalent organic frameworks for hydrogen peroxide photosynthesis. Nat. Commun. 14, 5238 (2023).
Google Scholar
Luo, Y. et al. Sulfone-modified covalent organic frameworks enabling efficient photocatalytic hydrogen peroxide generation via one-step two-electron O2 reduction. Angew. Chem. Int. Ed. 62, e202305355 (2023).
Google Scholar
Wang, H. et al. A crystalline partially fluorinated triazine covalent organic framework for efficient photosynthesis of hydrogen peroxide. Angew. Chem. Int. Ed. 61, e202202328 (2022).
Google Scholar
Delley, B. An all-electron numerical method for solving the local density functional for polyatomic molecules. J. Chem. Phys. 92, 508–517 (1990).
Google Scholar
Akkermans, R. L. C., Spenley, N. A. & Robertson, S. H. Monte Carlo methods in Materials Studio. Mol. Simul. 39, 1153–1164 (2013).
Google Scholar