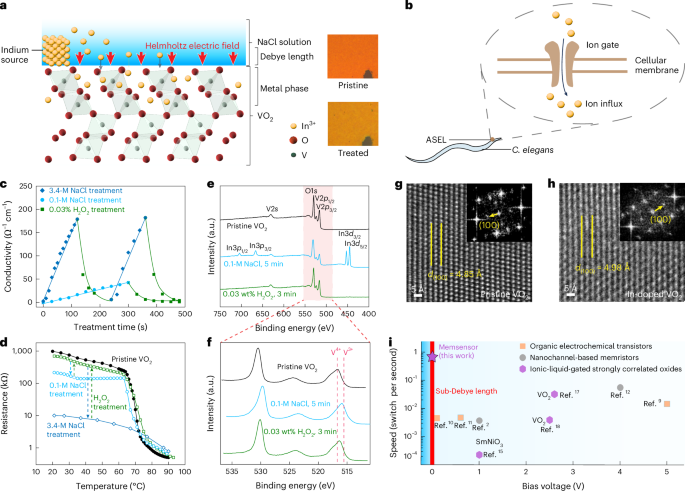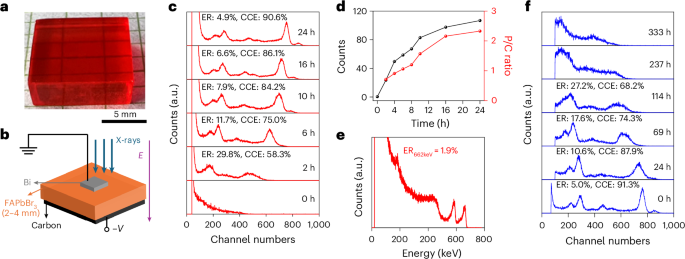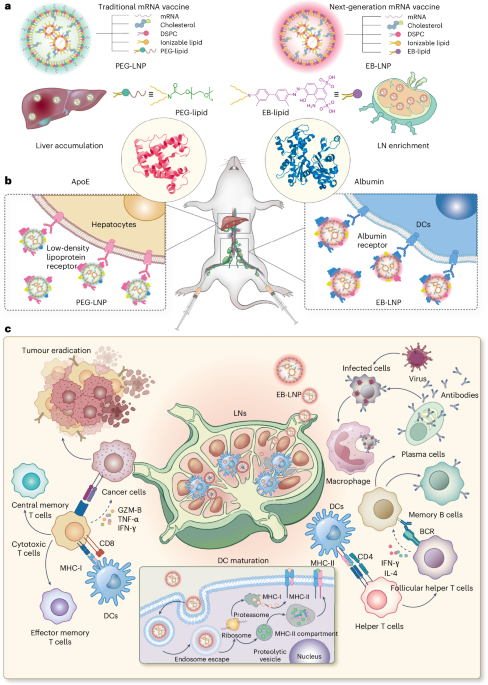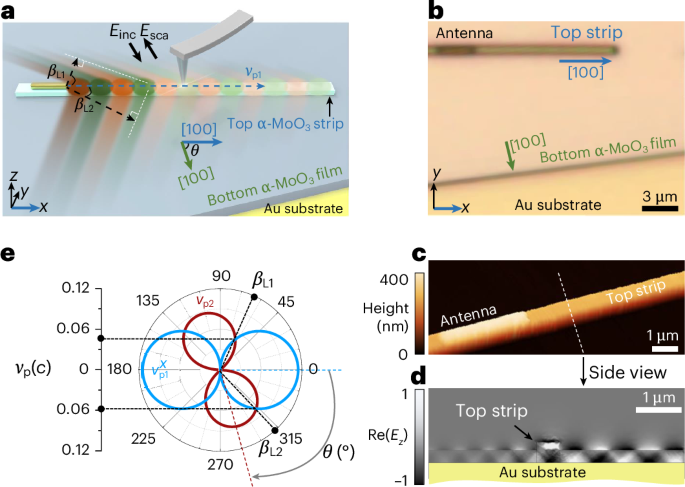
Barbier, A. J., Jiang, A. Y., Zhang, P., Wooster, R. & Anderson, D. G. The clinical progress of mRNA vaccines and immunotherapies. Nat. Biotechnol. 40, 840–854 (2022).
Google Scholar
Miao, L. et al. Delivery of mRNA vaccines with heterocyclic lipids increases anti-tumor efficacy by STING-mediated immune cell activation. Nat. Biotechnol. 37, 1174–1185 (2019).
Google Scholar
Liu, C. et al. mRNA-based cancer therapeutics. Nat. Rev. Cancer 23, 526–543 (2023).
Google Scholar
Rohner, E., Yang, R., Foo, K. S., Goedel, A. & Chien, K. R. Unlocking the promise of mRNA therapeutics. Nat. Biotechnol. 40, 1586–1600 (2022).
Google Scholar
Chabanovska, O., Galow, A. M., David, R. & Lemcke, H. mRNA—a game changer in regenerative medicine, cell-based therapy and reprogramming strategies. Adv. Drug Deliv. Rev. 179, 114002 (2021).
Google Scholar
Hou, X. C., Zaks, T., Langer, R. & Dong, Y. Z. Lipid nanoparticles for mRNA delivery. Nat. Rev. Mater. 7, 65–65 (2022).
Huang, X. G. et al. Nanotechnology-based strategies against SARS-CoV-2 variants. Nat. Nanotechnol. 17, 1027–1037 (2022).
Google Scholar
Akinc, A. et al. The Onpattro story and the clinical translation of nanomedicines containing nucleic acid-based drugs. Nat. Nanotechnol. 14, 1084–1087 (2019).
Google Scholar
Dahlman, J. E. & Loughrey, D. Non-liver mRNA delivery. Acc. Chem. Res. 55, 13–23 (2022).
Google Scholar
Dilliard, S. A. & Siegwart, D. J. Passive, active and endogenous organ-targeted lipid and polymer nanoparticles for delivery of genetic drugs. Nat. Rev. Mater. 8, 282–300 (2023).
Google Scholar
Cheng, Q. et al. Selective organ targeting (SORT) nanoparticles for tissue-specific mRNA delivery and CRISPR-Cas gene editing. Nat. Nanotechnol. 15, 313–316 (2020).
Google Scholar
Qiu, M. et al. Lung-selective mRNA delivery of synthetic lipid nanoparticles for the treatment of pulmonary lymphangioleiomyomatosis. Proc. Natl Acad. Sci. USA 119, e2116271119 (2022).
Google Scholar
Riley, R. S. et al. Ionizable lipid nanoparticles for in utero mRNA delivery. Sci. Adv. 7, eaba10 (2021).
Mitchell, M. J. et al. Ionizable lipid nanoparticles for in vivo mRNA Delivery to the placenta during pregnancy. J. Am. Chem. Soc. 145, 4691–4706 (2023).
Google Scholar
Liu, S. et al. Membrane-destabilizing ionizable phospholipids for organ-selective mRNA delivery and CRISPR-Cas gene editing. Nat. Mater. 20, 701–710 (2021).
Google Scholar
Dilliard, S. A., Cheng, Q. & Siegwart, D. J. On the mechanism of tissue-specific mRNA delivery by selective organ targeting nanoparticles. Proc. Natl Acad. Sci. USA 118, e2109256118 (2021).
Google Scholar
Poon, W., Kingston, B. R., Ouyang, B., Ngo, W. & Chan, W. C. W. A framework for designing delivery systems. Nat. Nanotechnol. 15, 819–829 (2020).
Google Scholar
Kedmi, R. et al. A modular platform for targeted RNAi therapeutics. Nat. Nanotechnol. 13, 214–219 (2018).
Google Scholar
Levin, A. et al. Biomimetic peptide self-assembly for functional materials. Nat. Rev. Chem. 4, 615–634 (2020).
Google Scholar
Huang, J. J. et al. Identification of potent antimicrobial peptides via a machine-learning pipeline that mines the entire space of peptide sequences. Nat. Biomed. Eng. 7, 797–810 (2023).
Google Scholar
Xie, J. et al. Cell-penetrating peptides in diagnosis and treatment of human diseases: from preclinical research to clinical application. Front. Pharmacol. 11, 697 (2020).
Google Scholar
Fosgerau, K. & Hoffmann, T. Peptide therapeutics: current status and future directions. Drug Discov. Today 20, 122–128 (2015).
Google Scholar
Foss, D. V. et al. Peptide-mediated delivery of CRISPR enzymes for the efficient editing of primary human lymphocytes. Nat. Biomed. Eng. 7, 647–660 (2023).
Google Scholar
Zhang, Z. et al. Efficient engineering of human and mouse primary cells using peptide-assisted genome editing. Nat. Biotechnol. 42, 305–315 (2023).
Google Scholar
Rhym, L. H., Manan, R. S., Koller, A., Stephanie, G. & Anderson, D. G. Peptide-encoding mRNA barcodes for the high-throughput in vivo screening of libraries of lipid nanoparticles for mRNA delivery. Nat. Biomed. Eng. 7, 901–910 (2023).
Google Scholar
Rossler, S. L., Grob, N. M., Buchwald, S. L. & Pentelute, B. L. Abiotic peptides as carriers of information for the encoding of small-molecule library synthesis. Science 379, eadf1354 (2023).
Huang, W. M. et al. Maleimide-thiol adducts stabilized through stretching. Nat. Chem. 11, 310–319 (2019).
Google Scholar
Stroock, A. et al. Chaotic mixer for microchannels. Science 295, 647–651 (2002).
Google Scholar
Li, S. D. & Huang, L. Stealth nanoparticles: high density but sheddable PEG is a key for tumor targeting. J. Control. Release 145, 178–181 (2010).
Google Scholar
Wang, S. et al. The role of protein corona on nanodrugs for organ-targeting and its prospects of application. J. Control. Release 360, 15–43 (2023).
Google Scholar
Oh, J. Y. et al. Cloaking nanoparticles with protein corona shield for targeted drug delivery. Nat. Commun. 9, 4548 (2018).
Google Scholar
Bashiri, G. et al. Nanoparticle protein corona: from structure and function to therapeutic targeting. Lab Chip 23, 1432–1466 (2023).
Google Scholar
Ngo, W. et al. Identifying cell receptors for the nanoparticle protein corona using genome screens. Nat. Chem. Biol. 18, 1023–1031 (2022).
Google Scholar
Singh, B., Fu, C. & Bhattacharya, J. Vascular expression of the αvβ3-integrin in lung and other organs. Am. J. Physiol. Lung Cell. Mol. Physiol. 278, L217–L226 (2000).
Google Scholar
Teoh, C., Tan, S. & Tran, T. Integrins as therapeutic targets for respiratory diseases. Curr. Mol. Med. 15, 714–734 (2015).
Google Scholar
Shanker, V. R., Bruun, T. U. J., Hie, B. L. & Kim, P. S. Unsupervised evolution of protein and antibody complexes with a structure-informed language model. Science 385, 46–53 (2024).
Google Scholar
Abramson, J. et al. Accurate structure prediction of biomolecular interactions with AlphaFold 3. Nature 630, 493–500 (2024).
Google Scholar
Schoenmaker, L. et al. mRNA-lipid nanoparticle COVID-19 vaccines: structure and stability. Int. J. Pharm. 601, 120586 (2021).
Google Scholar
Huang, X. G. et al. The landscape of mRNA nanomedicine. Nat. Med. 28, 2273–2287 (2022).
Google Scholar
Zhang, Y. B., Sun, C. Z., Wang, C., Jankovic, K. E. & Dong, Y. Z. Lipids and lipid derivatives for RNA delivery. Chem. Rev. 121, 12181–12277 (2021).
Google Scholar
Hajj, K. A. et al. A potent branched-tail lipid nanoparticle enables multiplexed mRNA delivery and gene editing in vivo. Nano Lett. 20, 5167–5175 (2020).
Google Scholar
Roberts, T. C., Langer, R. & Wood, M. J. A. Advances in oligonucleotide drug delivery. Nat. Rev. Drug Discov. 19, 673–694 (2020).
Google Scholar
Paunovska, K., Loughrey, D. & Dahlman, J. E. Drug delivery systems for RNA therapeutics. Nat. Rev. Genet. 23, 265–280 (2022).
Google Scholar
Yang, L. et al. Efficient delivery of antisense oligonucleotides using bioreducible lipid nanoparticles in vitro and in vivo. Mol. Ther. Nucleic Acids 19, 1357–1367 (2020).
Google Scholar
Liang, X. H. et al. Translation efficiency of mRNAs is increased by antisense oligonucleotides targeting upstream open reading frames. Nat. Biotechnol. 34, 875–880 (2016).
Google Scholar
Katti, A., Diaz, B. J., Caragine, C. M., Sanjana, N. E. & Dow, L. E. CRISPR in cancer biology and therapy. Nat. Rev. Cancer 22, 259–279 (2022).
Google Scholar
Platt, R. J. et al. CRISPR-Cas9 knockin mice for genome editing and cancer modeling. Cell 159, 440–455 (2014).
Google Scholar
Kim, G. B. et al. Rapid generation of somatic mouse mosaics with locus-specific, stably integrated transgenic elements. Cell 179, 251–267 (2019).
Google Scholar
Finn, J. D. et al. A single administration of CRISPR/Cas9 lipid nanoparticles achieves robust and persistent in vivo genome editing. Cell Rep. 22, 2227–2235 (2018).
Google Scholar
Zuris, J. A. et al. Cationic lipid-mediated delivery of proteins enables efficient protein-based genome editing in vitro and in vivo. Nat. Biotechnol. 33, 73–80 (2015).
Google Scholar
Wang, S. et al. Prostate-specific deletion of the murine Pten tumor suppressor gene leads to metastatic prostate cancer. Cancer Cell 4, 209–221 (2003).
Google Scholar
Rothgangl, T. et al. In vivo adenine base editing of PCSK9 in macaques reduces LDL cholesterol levels. Nat. Biotechnol. 39, 949–957 (2021).
Google Scholar
Donati, Y., Blaskovic, S., Ruchonnet-Métrailler, I., Lascano Maillard, J. & Barazzone-Argiroffo, C. Simultaneous isolation of endothelial and alveolar epithelial type I and type II cells during mouse lung development in the absence of a transgenic reporter. Am. J. Physiol. Lung Cell. Mol. Physiol. 318, L619–L630 (2020).
Google Scholar
Toulmin, S. A. et al. Type II alveolar cell MHCII improves respiratory viral disease outcomes while exhibiting limited antigen presentation. Nat. Commun. 12, 3993 (2021).
Google Scholar
Olesch, C. et al. Picturing of the lung tumor cellular composition by multispectral flow cytometry. Front. Immunol. 13, 827719 (2022).
Google Scholar
Sun, Y. et al. In vivo editing of lung stem cells for durable gene correction in mice. Science 384, 1196–1202 (2024).
Google Scholar
Modulation of CD19 surface expression in B cell acute lymphoblastic leukemia. Nat. Immunol. 23, 1410–1411 (2022).
Swingle, K. L. et al. Placenta-tropic VEGF mRNA lipid nanoparticles ameliorate murine pre-eclampsia. Nature https://doi.org/10.1038/s41586-024-08291-2 (2024).
Google Scholar
Lian, X. et al. Bone-marrow-homing lipid nanoparticles for genome editing in diseased and malignant haematopoietic stem cells. Nat. Nanotechnol. 19, 1409–1417 (2024).
Google Scholar
Adolfsson, J. et al. Upregulation of Flt3 expression within the bone marrow Lin−Sca1+c-kit+ stem cell compartment is accompanied by loss of self-renewal capacity. Immunity 15, 659–669 (2001).
Google Scholar
Engelhard, S. et al. Endomucin marks quiescent long-term multi-lineage repopulating hematopoietic stem cells and is essential for their transendothelial migration. Cell Rep. 43, 114475 (2024).
Google Scholar
Mayle, A., Luo, M., Jeong, M. & Goodell, M. A. Flow cytometry analysis of murine hematopoietic stem cells. Cytom. Part A 83A, 27–37 (2013).
Google Scholar
Rodeheffer, M. S., Birsoy, K. & Friedman, J. M. Identification of white adipocyte progenitor cells in vivo. Cell 135, 240–249 (2008).
Google Scholar
Lee, Y.-H., Petkova, A. P., Mottillo, E. P. & Granneman, J. G. In vivo identification of bipotential adipocyte progenitors recruited by β3-adrenoceptor activation and high-fat feeding. Cell Metab. 15, 480–491 (2012).
Google Scholar
Garritson, J. D. et al. BMPER is a marker of adipose progenitors and adipocytes and a positive modulator of adipogenesis. Commun. Biol. 6, 638 (2023).
Google Scholar
Zomer, H. D. & Reddi, P. P. Characterization of rodent Sertoli cell primary cultures. Mol. Reprod. Dev. 87, 857–870 (2020).
Google Scholar
Song, Y., Zhang, X., Desmarais, J. A. & Nagano, M. Postnatal development of mouse spermatogonial stem cells as determined by immunophenotype, regenerative capacity, and long-term culture-initiating ability: a model for practical applications. Sci. Rep. 14, 2299 (2024).
Google Scholar
Omo-Lamai, S. et al. Physicochemical targeting of lipid nanoparticles to the lungs induces clotting: mechanisms and solutions. Adv. Mater. 36, 2312026 (2023).
Witten, J. et al. Artificial intelligence-guided design of lipid nanoparticles for pulmonary gene therapy. Nat. Biotechnol. https://doi.org/10.1038/s41587-024-02490-y (2024).
Google Scholar
Li, B. et al. Accelerating ionizable lipid discovery for mRNA delivery using machine learning and combinatorial chemistry. Nat. Mater. 23, 1002–1008 (2024).
Google Scholar
Dobrowolski, C. et al. Nanoparticle single-cell multiomic readouts reveal that cell heterogeneity influences lipid nanoparticle-mediated messenger RNA delivery. Nat. Nanotechnol. 17, 871–879 (2022).
Google Scholar
Phillips, J. C. et al. Scalable molecular dynamics on CPU and GPU architectures with NAMD. J. Chem. Phys. 153, 044130 (2020).
Google Scholar
Best, R. B. et al. Optimization of the additive CHARMM all-atom protein force field targeting improved sampling of the backbone phi, psi and side-chain χ1 and χ2 dihedral angles. J. Chem. Theory Comput. 8, 3257–3273 (2012).
Google Scholar
Jorgensen, W. L., Chandrasekhar, J., Madura, J. D., Impey, R. W. & Klein, M. L. Comparison of simple potential functions for simulating liquid water. J. Chem. Phys. 79, 926–935 (1983).
Google Scholar
Sali, A. & Blundell, T. L. Comparative protein modeling by satisfaction of spatial restraints. J. Mol. Biol. 234, 779–815 (1993).
Google Scholar
Abdin, O., Nim, S., Wen, H. & Kim, P. M. PepNN: a deep attention model for the identification of peptide binding sites. Commun. Biol. 5, 503 (2022).
Google Scholar
Kozlovskii, I. & Popov, P. Protein-peptide binding site detection using 3D convolutional neural networks. J. Chem. Inf. Model. 61, 3814–3823 (2021).
Google Scholar
Valdés-Tresanco, M. S., Valdés-Tresanco, M. E., Valiente, P. A. & Moreno, E. gmx_MMPBSA: a new tool to perform end-state free energy calculations with GROMACS. J. Chem. Theory Comput. 17, 6281–6291 (2021).
Google Scholar
Miller, B. R. III et al. MMPBSA.py: an efficient program for end-state free energy calculations. J. Chem. Theory Comput. 8, 3314–3321 (2012).
Google Scholar