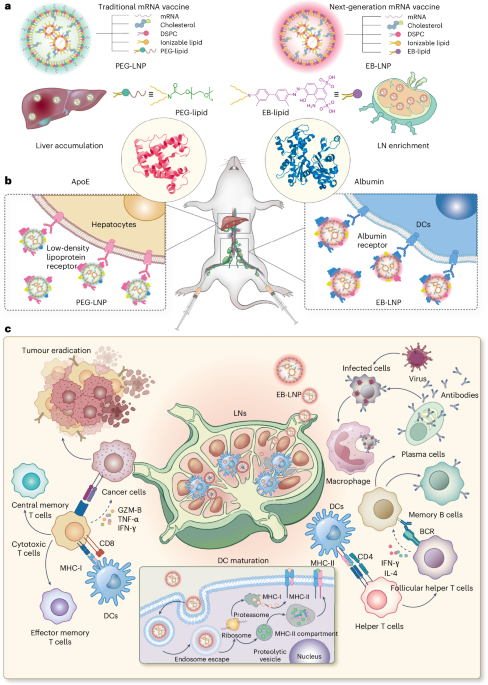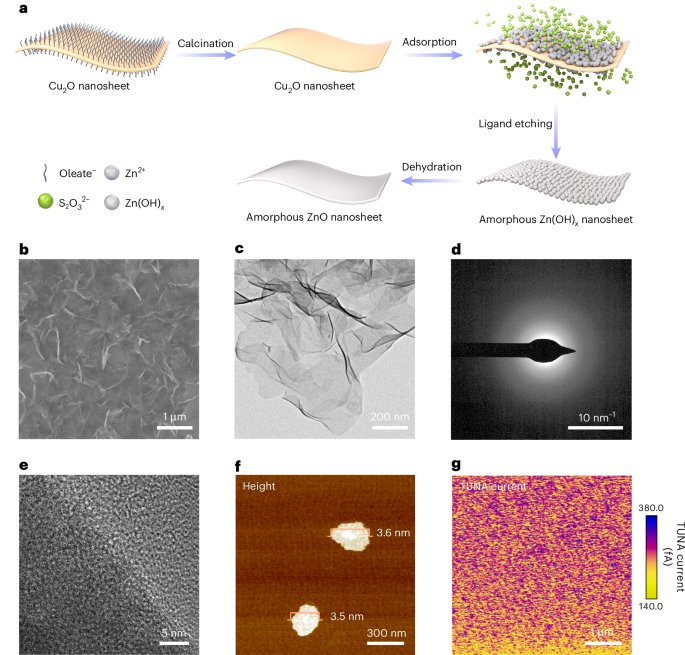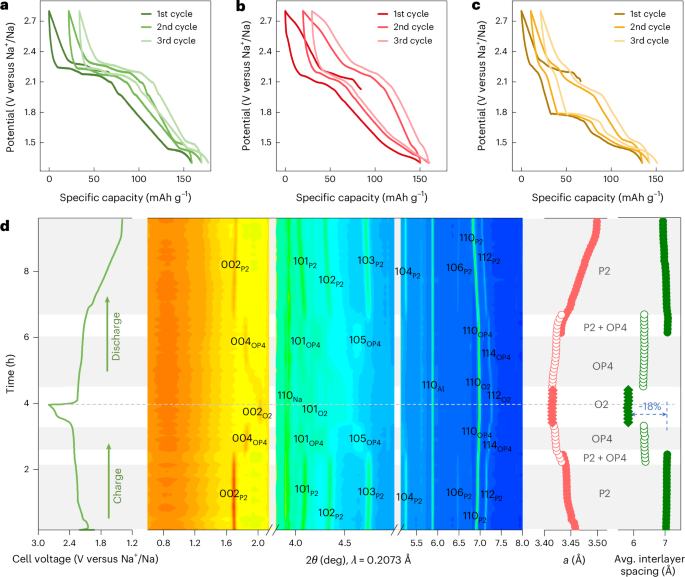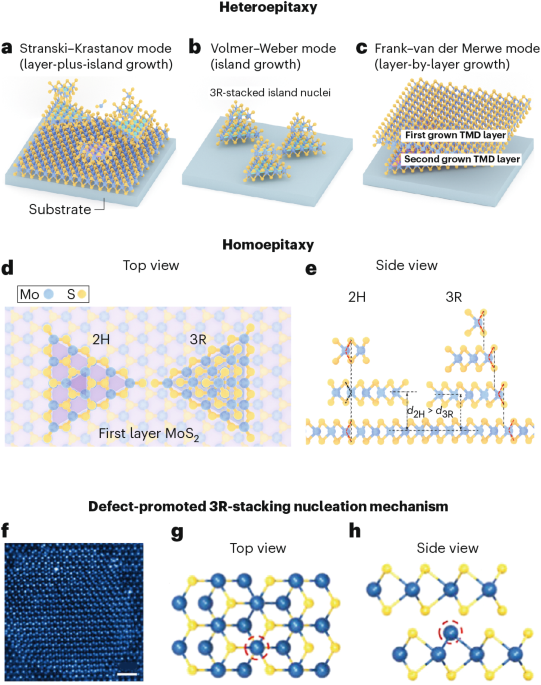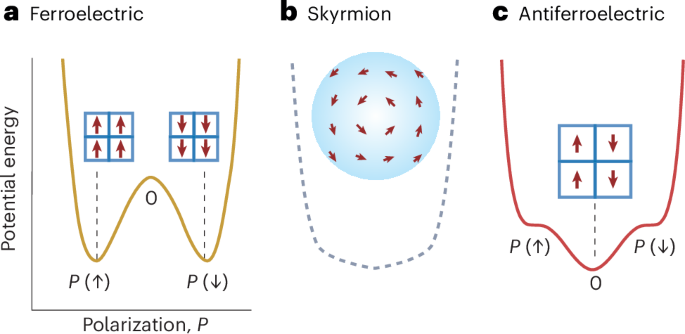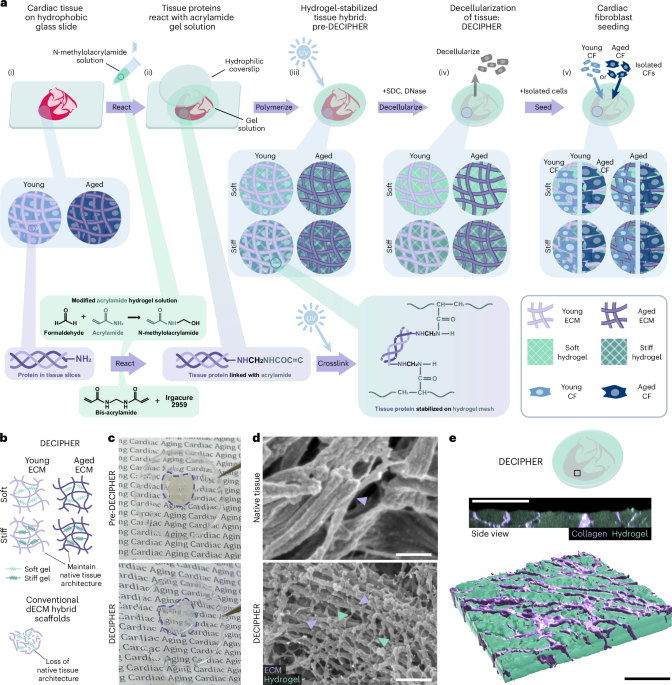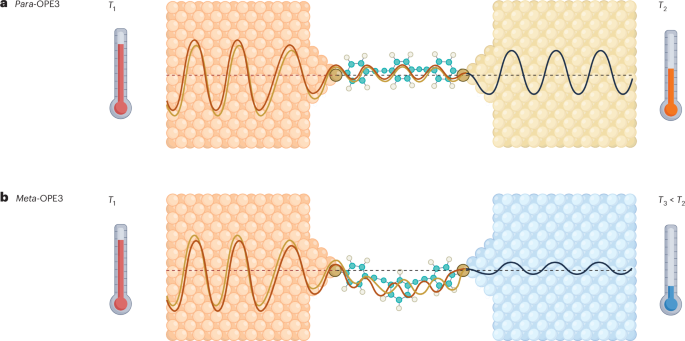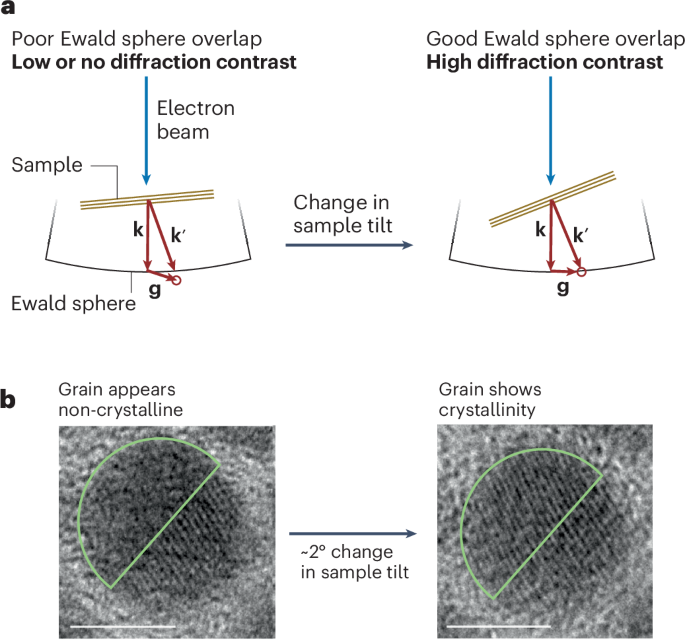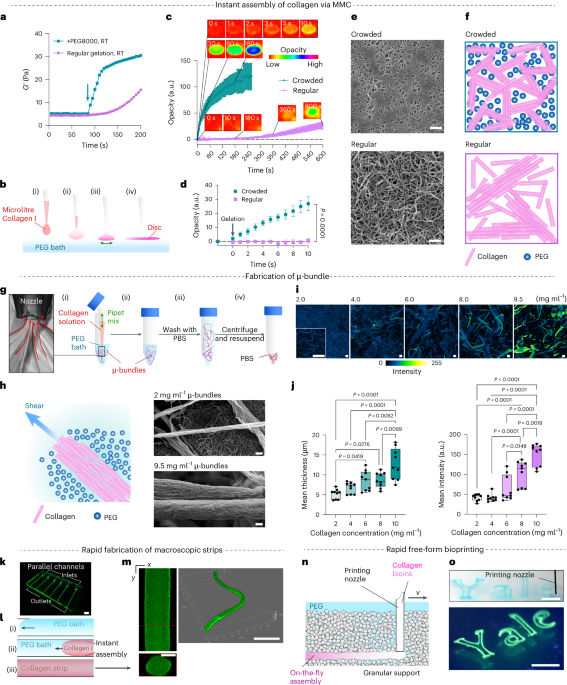
Cullis, P. R. & Hope, M. J. Lipid nanoparticle systems for enabling gene therapies. Mol. Ther. 25, 1467–1475 (2017).
Google Scholar
Mullard, A. COVID-19 vaccine success enables a bolder vision for mRNA cancer vaccines, says BioNTech CEO. Nat. Rev. Drug Discov. 20, 500–501 (2021).
Google Scholar
Hou, X., Zaks, T., Langer, R. & Dong, Y. Lipid nanoparticles for mRNA delivery. Nat. Rev. Mater. 6, 1078–1094 (2021).
Google Scholar
Akinc, A. et al. The Onpattro story and the clinical translation of nanomedicines containing nucleic acid-based drugs. Nat. Nanotechnol. 14, 1084–1087 (2019).
Google Scholar
Hassett, K. J. et al. Optimization of lipid nanoparticles for intramuscular administration of mRNA vaccines. Mol. Ther. Nucleic Acids 15, 1–11 (2019).
Google Scholar
Schoenmaker, L. et al. mRNA-lipid nanoparticle COVID-19 vaccines: structure and stability. Int. J. Pharm. 601, 120586 (2021).
Google Scholar
Zhang, P., Sun, F., Liu, S. & Jiang, S. Anti-PEG antibodies in the clinic: current issues and beyond PEGylation. J. Control. Release 244, 184–193 (2016).
Google Scholar
Kumar, V. et al. Shielding of lipid nanoparticles for siRNA delivery: impact on physicochemical properties, cytokine induction, and efficacy. Mol. Ther. Nucleic Acids 3, e210 (2014).
Google Scholar
Yang, Q. et al. Analysis of pre-existing IgG and IgM antibodies against polyethylene glycol (PEG) in the general population. Anal. Chem. 88, 11804–11812 (2016).
Google Scholar
Besin, G. et al. Accelerated blood clearance of lipid nanoparticles entails a biphasic humoral response of B-1 followed by B-2 lymphocytes to distinct antigenic moieties. Immunohorizons 3, 282–293 (2019).
Google Scholar
Sanchez, A. et al. Substituting poly(ethylene glycol) lipids with poly(2-ethyl-2-oxazoline) lipids improves lipid nanoparticle repeat dosing. Adv. Health. Mater. 13, e2304033 (2024).
Google Scholar
Ju, Y. et al. Anti-PEG antibodies boosted in humans by SARS-CoV-2 lipid nanoparticle mRNA vaccine. ACS Nano 16, 11769–11780 (2022).
Google Scholar
Vrieze, J. Suspicions grow that nanoparticles in Pfizer’s COVID-19 vaccine trigger rare allergic reactions. Science https://www.science.org/content/article/suspicions-grow-nanoparticles-pfizer-s-covid-19-vaccine-trigger-rare-allergic-reactions (21 December 2020).
Kang, D. D. et al. Engineering LNPs with polysarcosine lipids for mRNA delivery. Bioact. Mater. 37, 86–93 (2024).
Google Scholar
Nogueira, S. S. et al. Polysarcosine-functionalized lipid nanoparticles for therapeutic mRNA delivery. ACS Appl. Nano Mater. 3, 10634–10645 (2020).
Google Scholar
Cao, Z., Zhang, L. & Jiang, S. Superhydrophilic zwitterionic polymers stabilize liposomes. Langmuir 28, 11625–11632 (2012).
Google Scholar
Li, Y. et al. Enhanced endosomal/lysosomal escape by distearoyl phosphoethanolamine-polycarboxybetaine lipid for systemic delivery of siRNA. J. Control. Release 176, 104–114 (2014).
Google Scholar
Li, Q. et al. Zwitterionic biomaterials. Chem. Rev. 122, 17073–17154 (2022).
Google Scholar
Li, B. et al. Zwitterionic nanocages overcome the efficacy loss of biologic drugs. Adv. Mater. 30, e1705728 (2018).
Google Scholar
Keefe, A. J. & Jiang, S. Poly(zwitterionic)protein conjugates offer increased stability without sacrificing binding affinity or bioactivity. Nat. Chem. 4, 59–63 (2011).
Google Scholar
Li, B. et al. Revealing the immunogenic risk of polymers. Angew. Chem. Int. Ed. 57, 13873–13876 (2018).
Google Scholar
Wang, X. et al. Preparation of selective organ-targeting (SORT) lipid nanoparticles (LNPs) using multiple technical methods for tissue-specific mRNA delivery. Nat. Protoc. 18, 265–291 (2023).
Google Scholar
Dong, Y. et al. Lipopeptide nanoparticles for potent and selective siRNA delivery in rodents and nonhuman primates. Proc. Natl Acad. Sci. USA 111, 3955–3960 (2014).
Google Scholar
Kumar, A. R. K., Shou, Y., Chan, B., L, K. & Tay, A. Materials for improving immune cell transfection. Adv. Mater. 33, e2007421 (2021).
Google Scholar
Patel, S. et al. Beyond CAR T cells: other cell-based immunotherapeutic strategies against cancer. Front. Oncol. 9, 196 (2019).
Google Scholar
Weber, E. W., Maus, M. V. & Mackall, C. L. The emerging landscape of immune cell therapies. Cell 181, 46–62 (2020).
Google Scholar
Jahan, F. et al. Using the Jurkat reporter T cell line for evaluating the functionality of novel chimeric antigen receptors. Front. Mol. Med. 3, 1070384 (2023).
Google Scholar
Chang, Y. et al. CAR-neutrophil mediated delivery of tumor-microenvironment responsive nanodrugs for glioblastoma chemo-immunotherapy. Nat. Commun. 14, 2266 (2023).
Google Scholar
Ren, J. et al. Multiplex genome editing to generate universal CAR T cells resistant to PD1 inhibition. Clin. Cancer Res. 23, 2255–2266 (2017).
Google Scholar
Barbier, A. J., Jiang, A. Y., Zhang, P., Wooster, R. & Anderson, D. G. The clinical progress of mRNA vaccines and immunotherapies. Nat. Biotechnol. 40, 840–854 (2022).
Google Scholar
Kreiter, S. et al. Increased antigen presentation efficiency by coupling antigens to MHC class I trafficking signals. J. Immunol. 180, 309–318 (2008).
Google Scholar
Yanez Arteta, M. et al. Successful reprogramming of cellular protein production through mRNA delivered by functionalized lipid nanoparticles. Proc. Natl Acad. Sci. USA 115, E3351–E3360 (2018).
Google Scholar
Aburai, K., Hatanaka, K., Takano, S., Fujii, S. & Sakurai, K. Characterizing an siRNA-containing lipid-nanoparticle prepared by a microfluidic reactor: small-angle X-ray scattering and cryotransmission electron microscopic studies. Langmuir 36, 12545–12554 (2020).
Google Scholar
Patel, S. et al. Naturally-occurring cholesterol analogues in lipid nanoparticles induce polymorphic shape and enhance intracellular delivery of mRNA. Nat. Commun. 11, 983 (2020).
Google Scholar
Cornebise, M. et al. Discovery of a novel amino lipid that improves lipid nanoparticle performance through specific interactions with mRNA. Adv. Funct. Mater. 32, 2106727 (2022).
Google Scholar
Leung, A. K. et al. Lipid nanoparticles containing siRNA synthesized by microfluidic mixing exhibit an electron-dense nanostructured core. J. Phys. Chem. C 116, 18440–18450 (2012).
Google Scholar
Cheng, M. H. Y. et al. Induction of bleb structures in lipid nanoparticle formulations of mRNA leads to improved transfection potency. Adv. Mater. 35, e2303370 (2023).
Google Scholar
Dammes, N. et al. Conformation-sensitive targeting of lipid nanoparticles for RNA therapeutics. Nat. Nanotechnol. 16, 1030–1038 (2021).
Google Scholar
Sedic, M. et al. Safety evaluation of lipid nanoparticle-formulated modified mRNA in the Sprague-Dawley rat and cynomolgus monkey. Vet. Pathol. 55, 341–354 (2018).
Google Scholar
Vlatkovic, I. Non-immunotherapy application of LNP-mRNA: maximizing efficacy and safety. Biomedicines 9, 530 (2021).
Google Scholar
Liang, F. et al. Efficient targeting and activation of antigen-presenting cells in vivo after modified mRNA vaccine administration in rhesus macaques. Mol. Ther. 25, 2635–2647 (2017).
Google Scholar
Chaudhary, N. et al. Amine headgroups in ionizable lipids drive immune responses to lipid nanoparticles by binding to the receptors TLR4 and CD1d. Nat. Biomed. Eng 8, 1483–1498 (2024).
Google Scholar
Schlich, M. et al. Cytosolic delivery of nucleic acids: the case of ionizable lipid nanoparticles. Bioeng Transl. Med. 6, e10213 (2021).
Google Scholar
Bruininks, B. M., Souza, P. C., Ingolfsson, H. & Marrink, S. J. A molecular view on the escape of lipoplexed DNA from the endosome. eLife 9, e52012 (2020).
Google Scholar
Liu, S. & Jiang, S. Zwitterionic polymer-protein conjugates reduce polymer-specific antibody response. Nano Today 11, 285–291 (2016).
Google Scholar
Kochenderfer, J. N. et al. Eradication of B-lineage cells and regression of lymphoma in a patient treated with autologous T cells genetically engineered to recognize CD19. Blood 116, 4099–4102 (2010).
Google Scholar
Henderson, J. M. et al. Cap 1 messenger RNA synthesis with co-transcriptional CleanCap® analog by in vitro transcription. Curr. Protoc. 1, e39 (2021).
Google Scholar
Nance, K. D. & Meier, J. L. Modifications in an emergency: the role of N1-methylpseudouridine in COVID-19 vaccines. ACS Cent. Sci. 7, 748–756 (2021).
Google Scholar
Hopkins, J. B., Gillilan, R. E. & Skou, S. BioXTAS RAW: improvements to a free open-source program for small-angle X-ray scattering data reduction and analysis. J. Appl. Crystallogr. 50, 1545–1553 (2017).
Google Scholar
Luozhong, S. et al. Phosphatidylserine lipid nanoparticles promote systemic RNA delivery to secondary lymphoid organs. Nano Lett. 22, 8304–8311 (2022).
Google Scholar
Nelson, J. et al. Impact of mRNA chemistry and manufacturing process on innate immune activation. Sci. Adv. 6, eaaz6893 (2020).
Google Scholar
Baiersdorfer, M. et al. A facile method for the removal of dsRNA contaminant from in vitro-transcribed mRNA. Mol. Ther. Nucleic Acids 15, 26–35 (2019).
Google Scholar