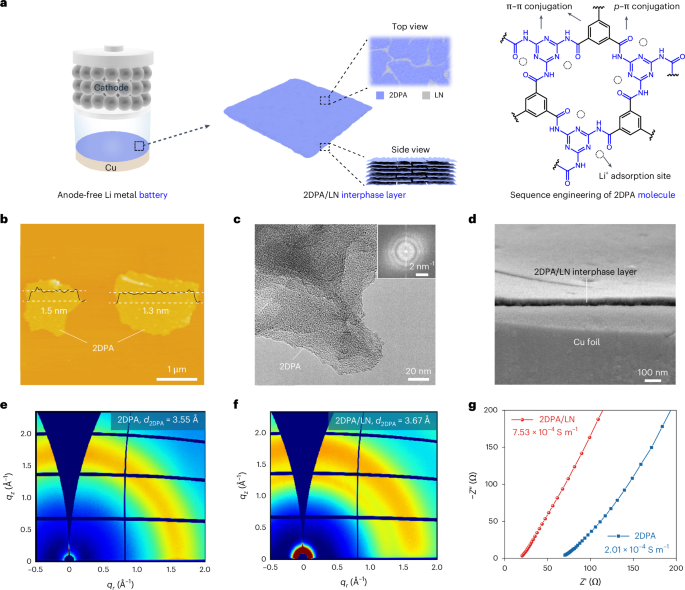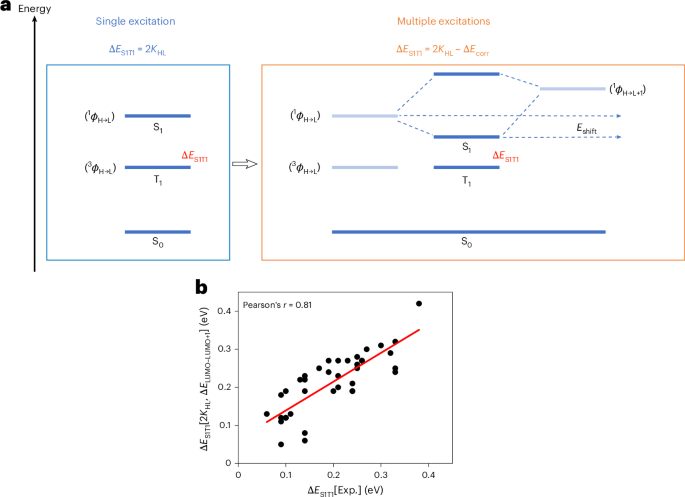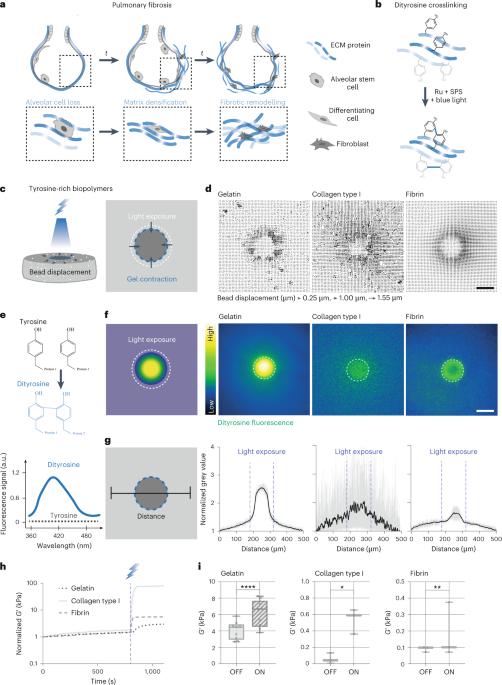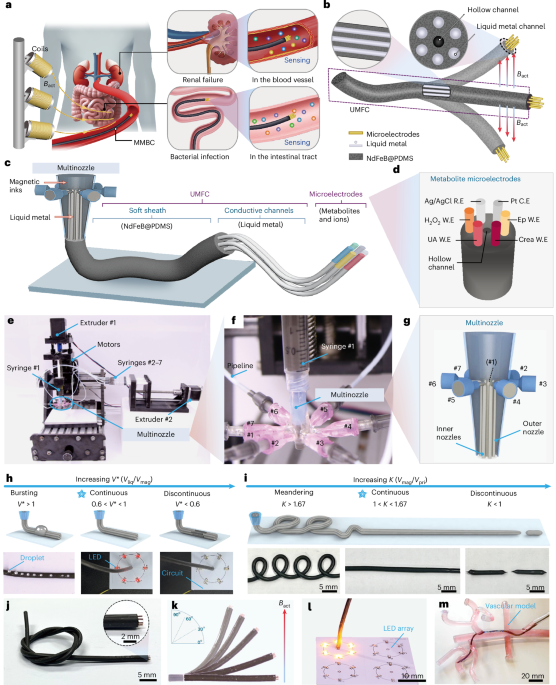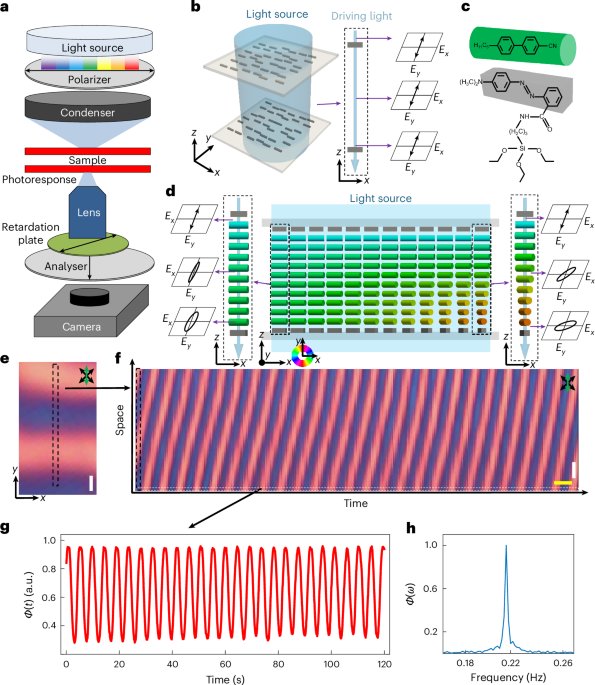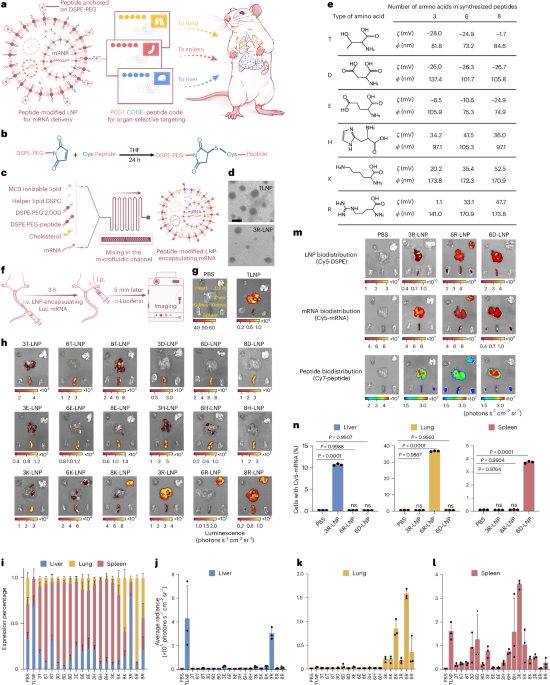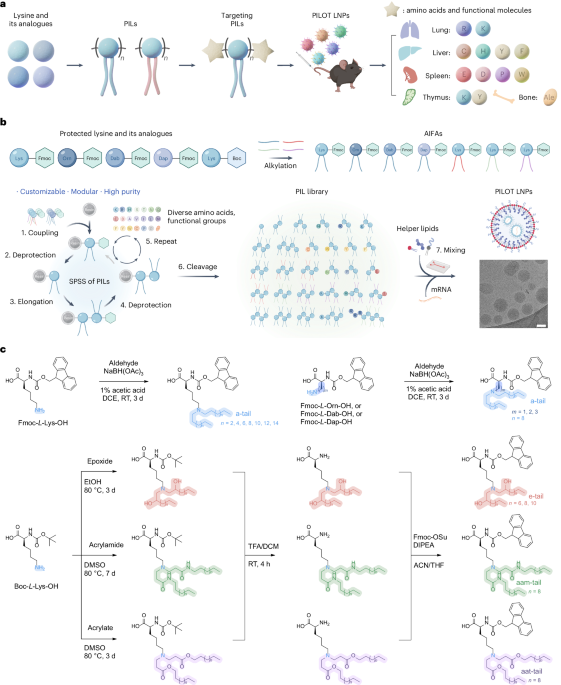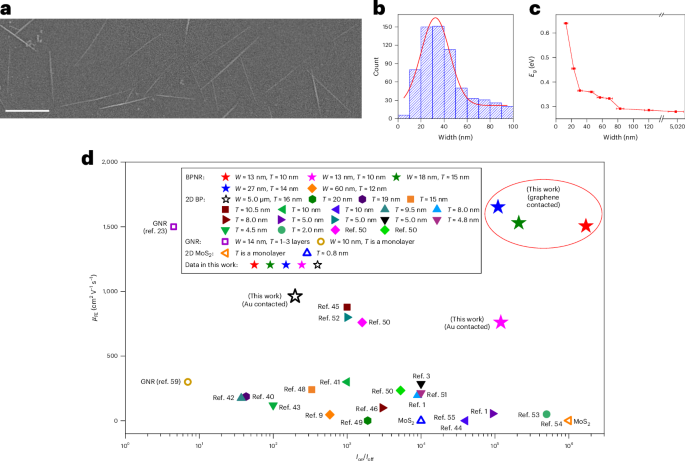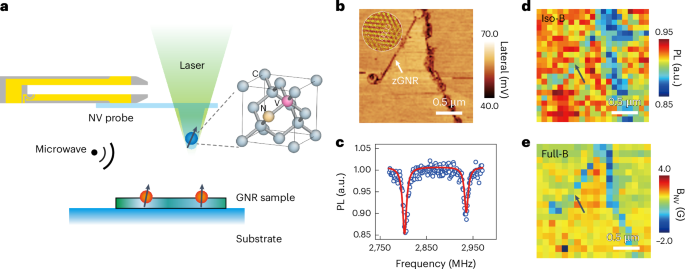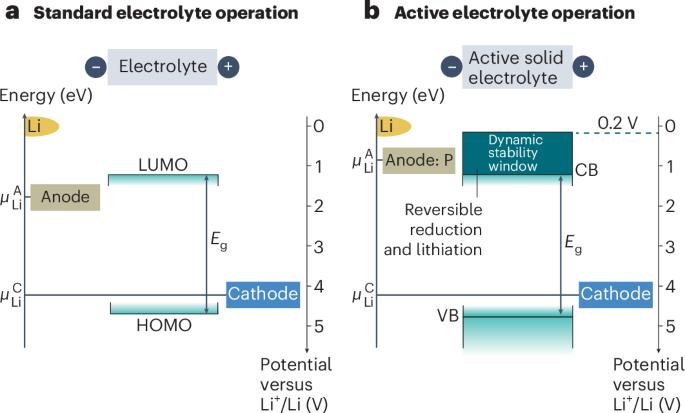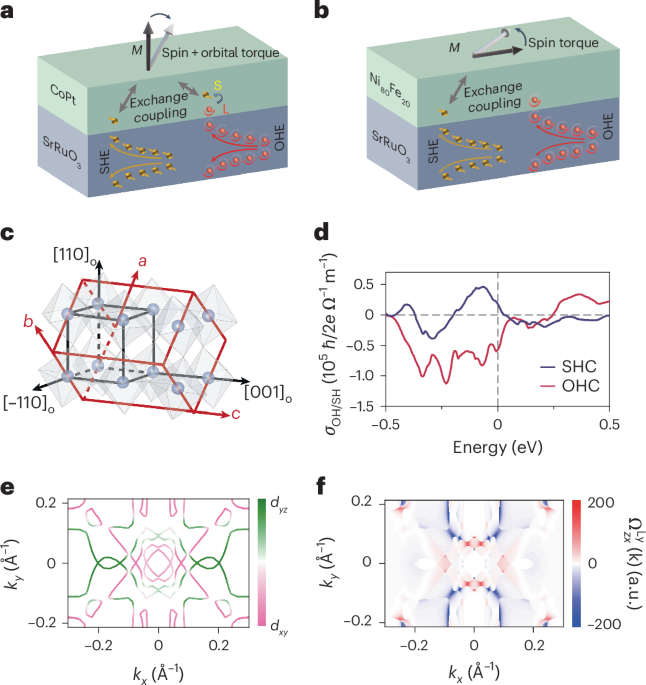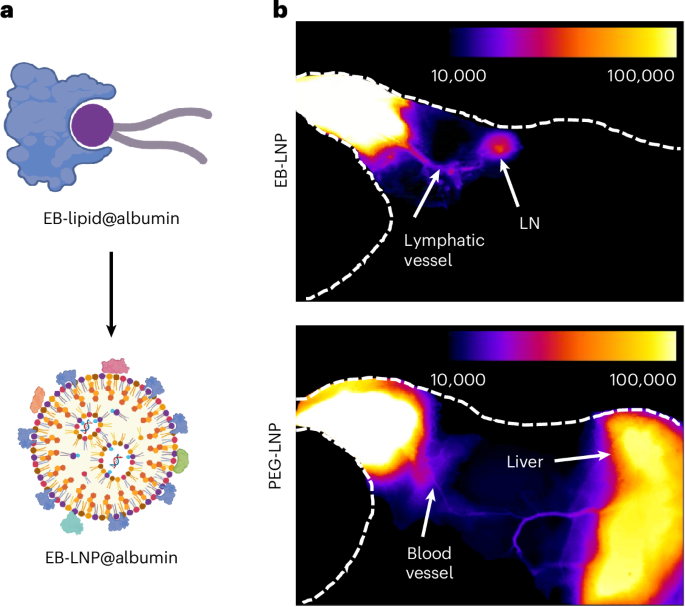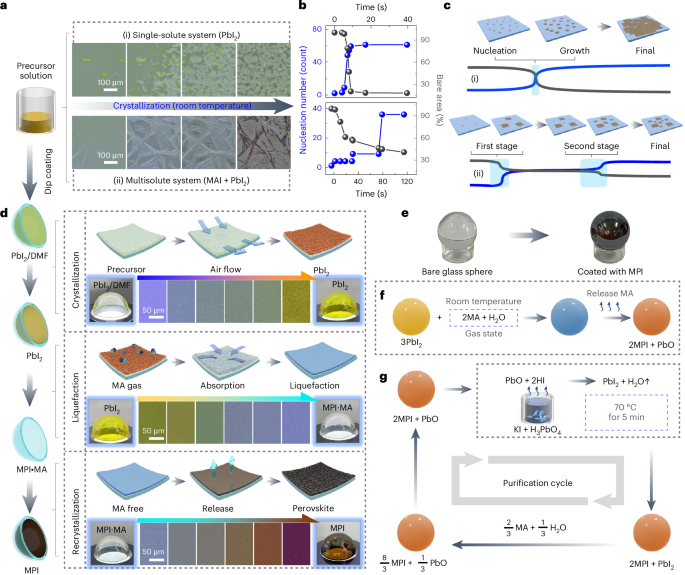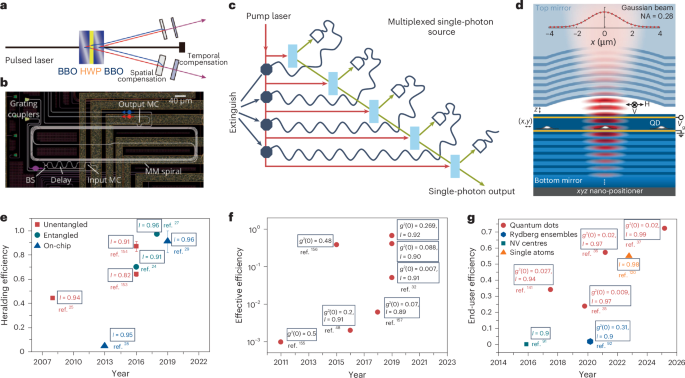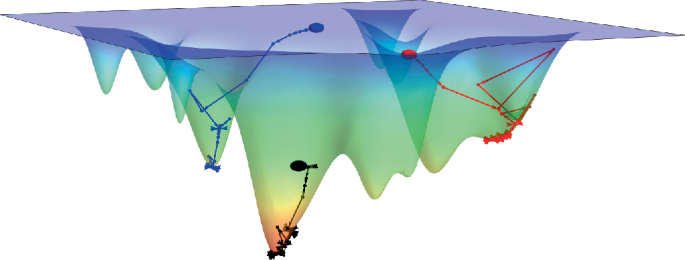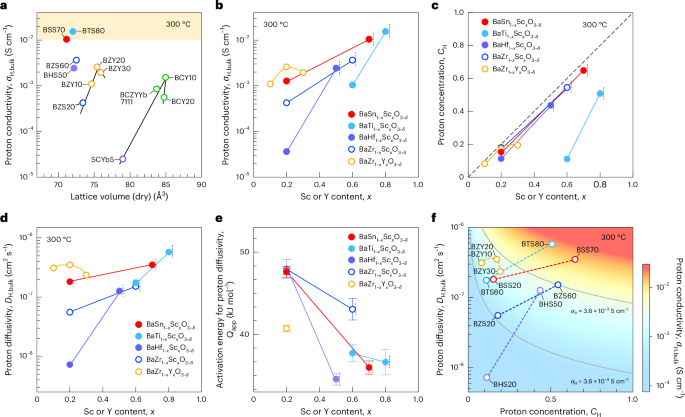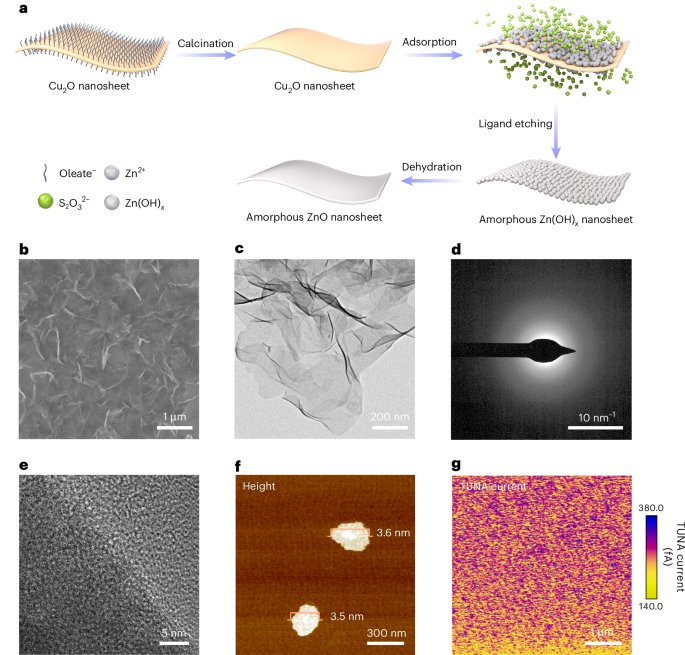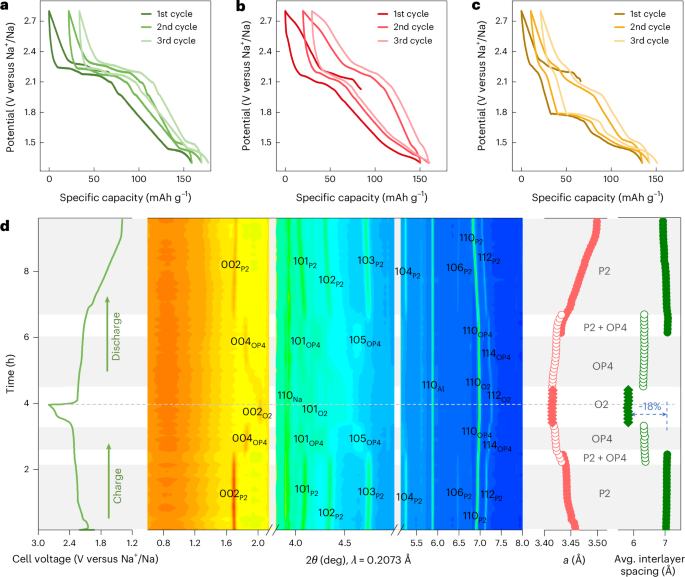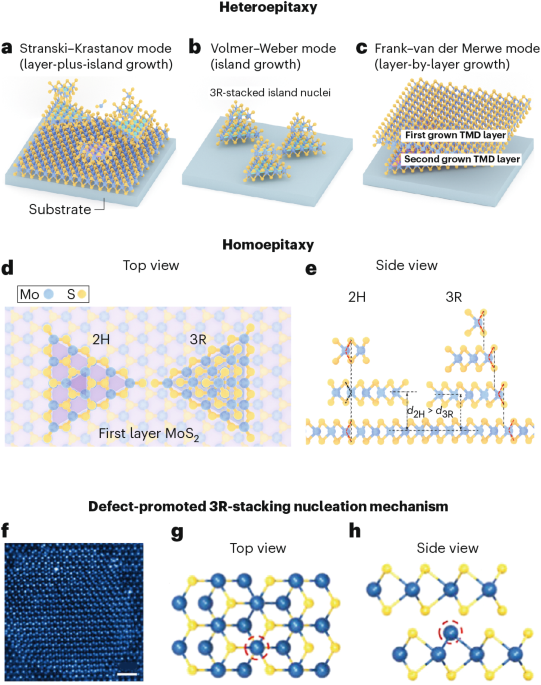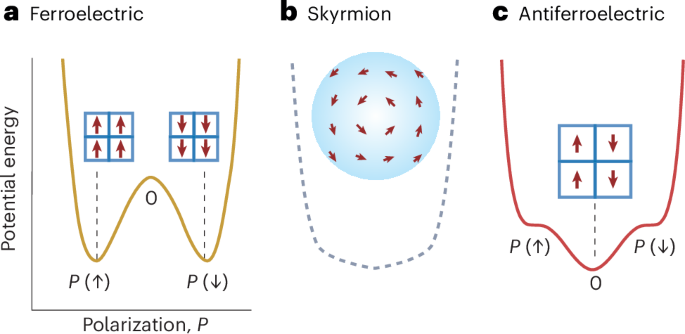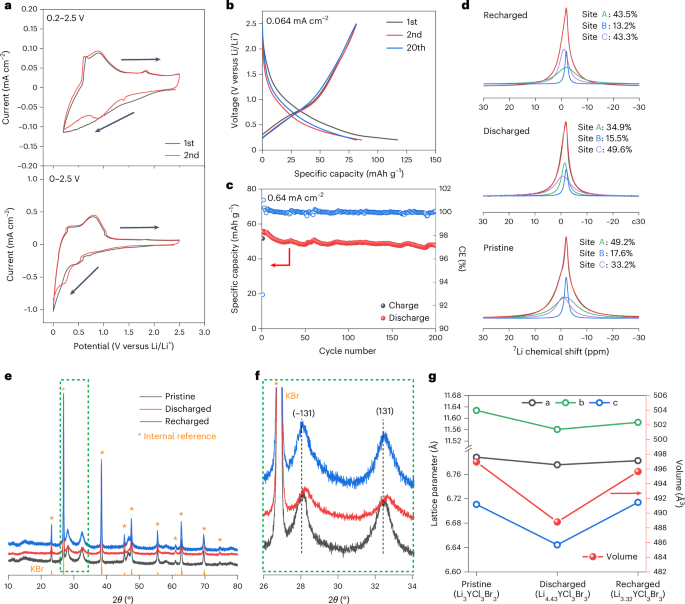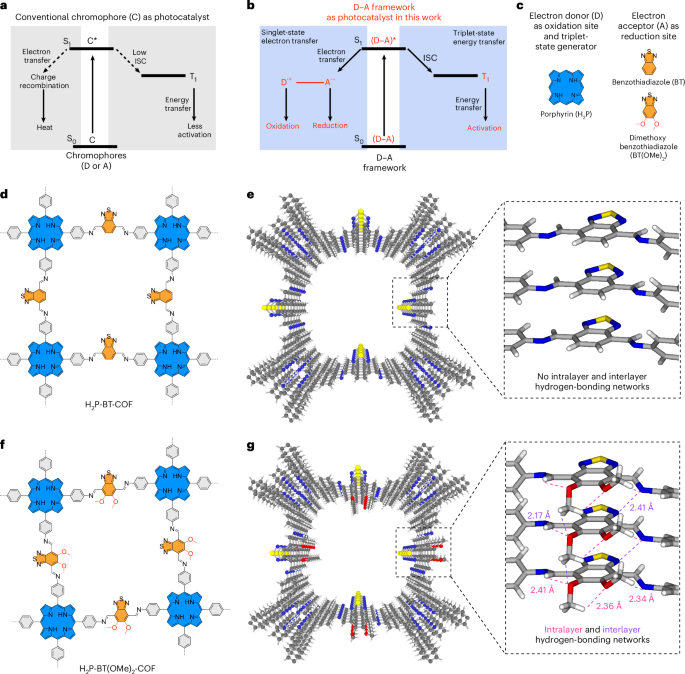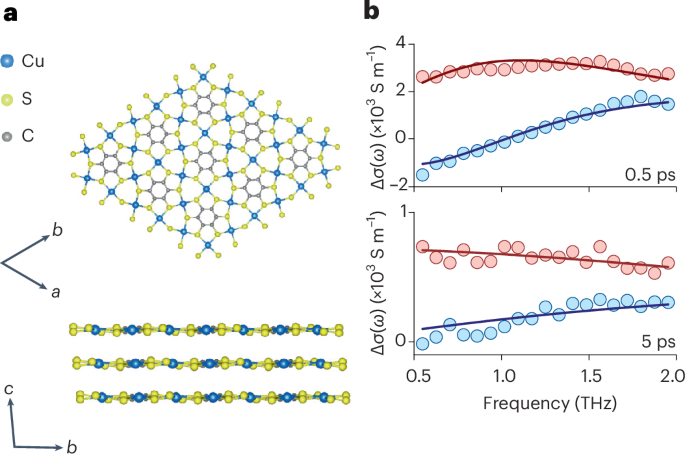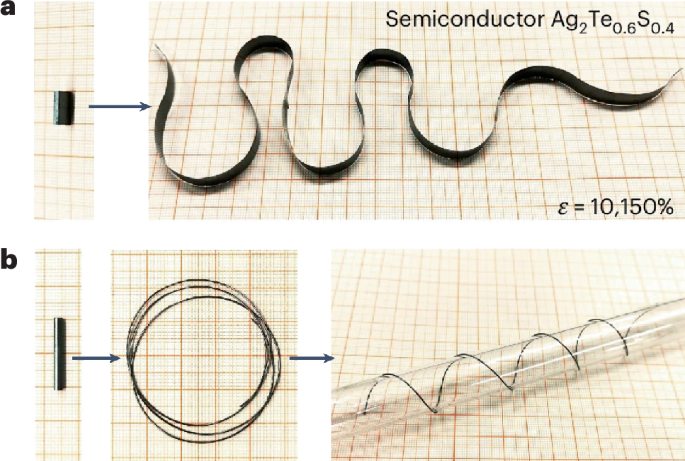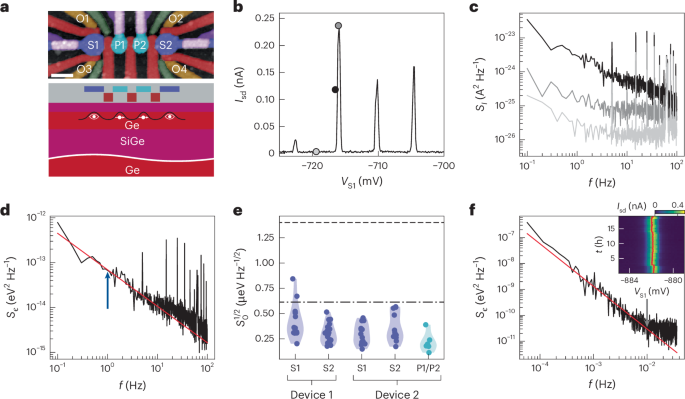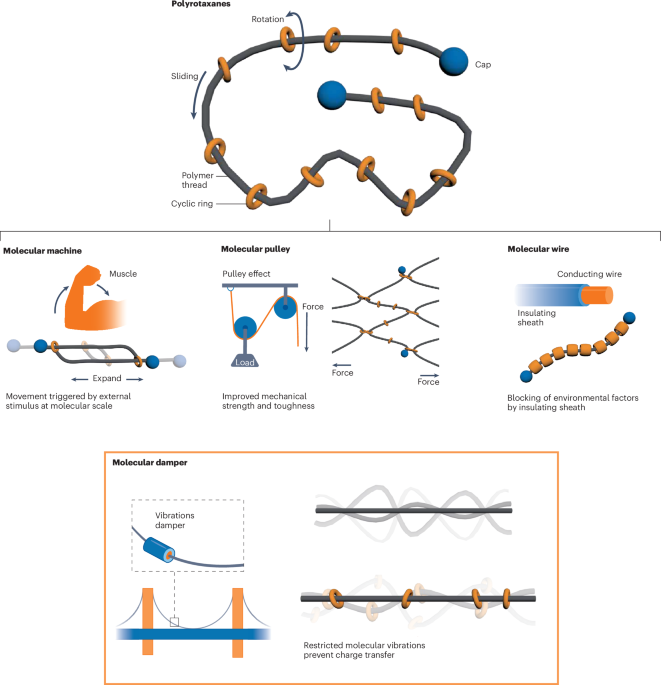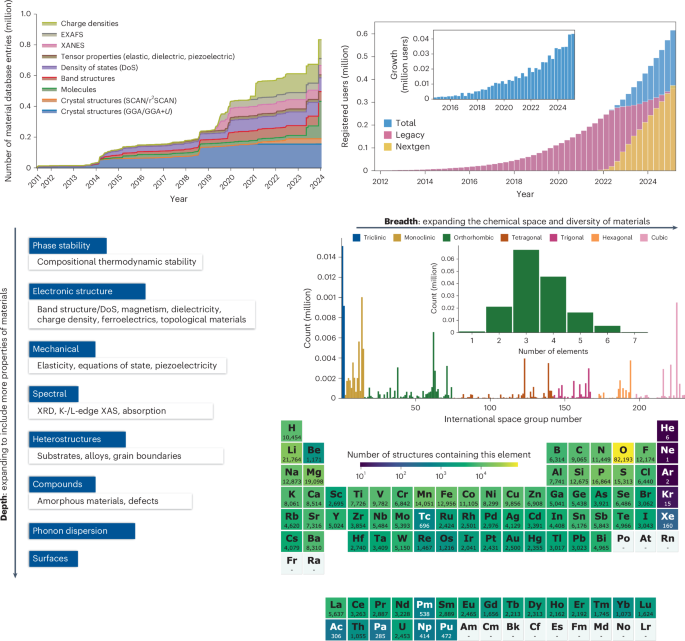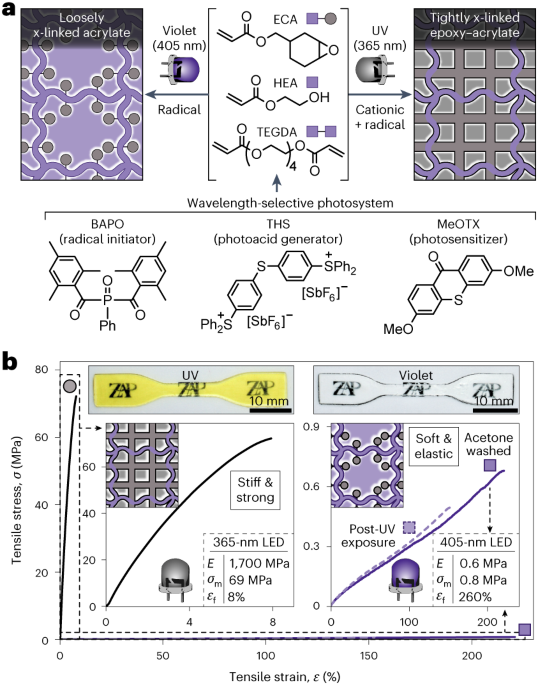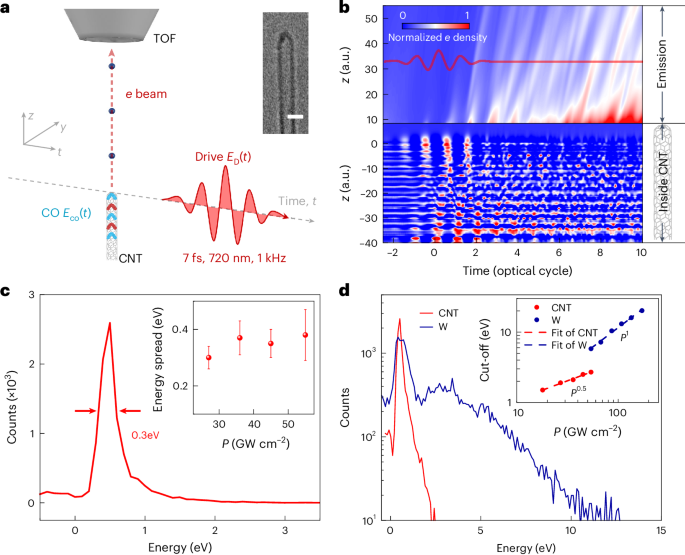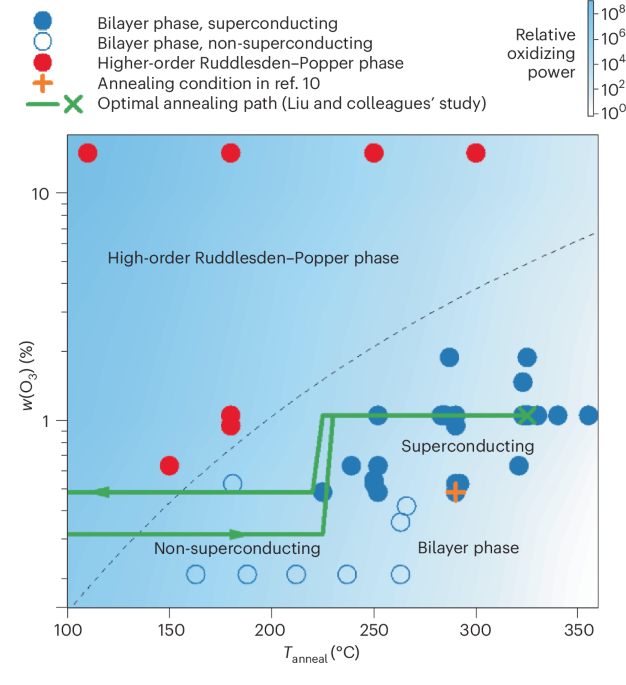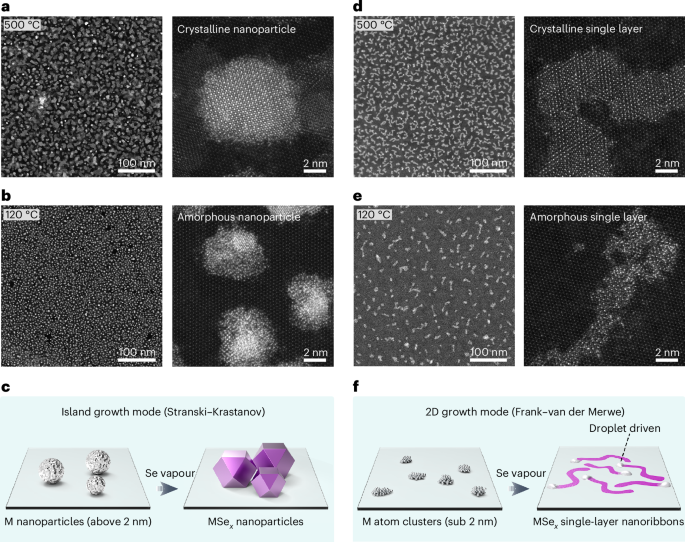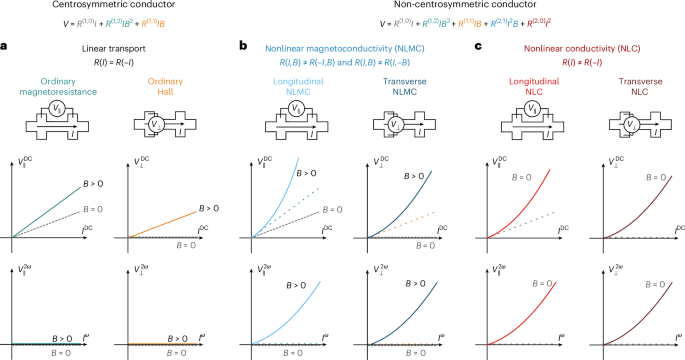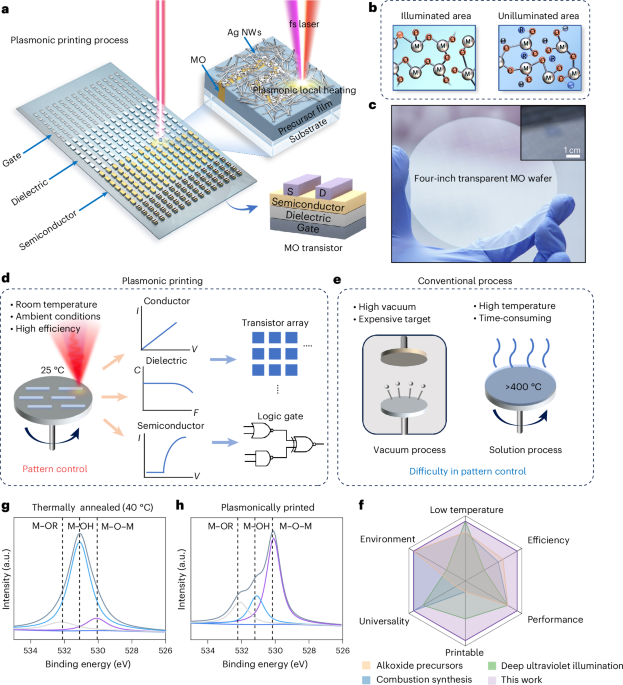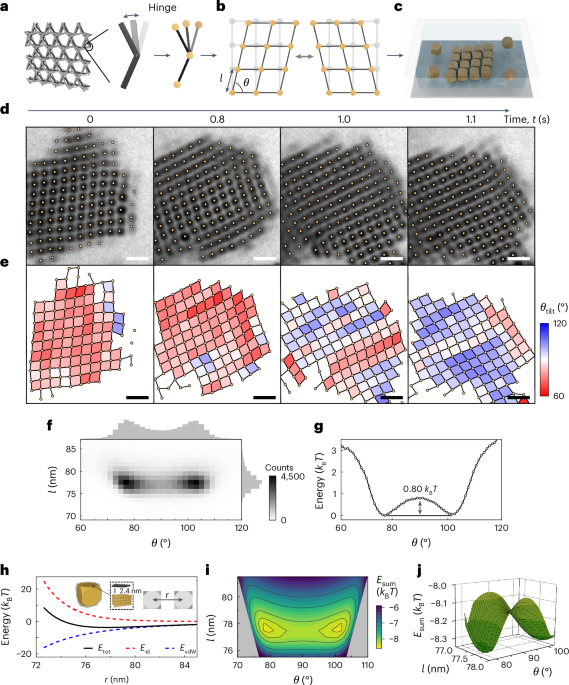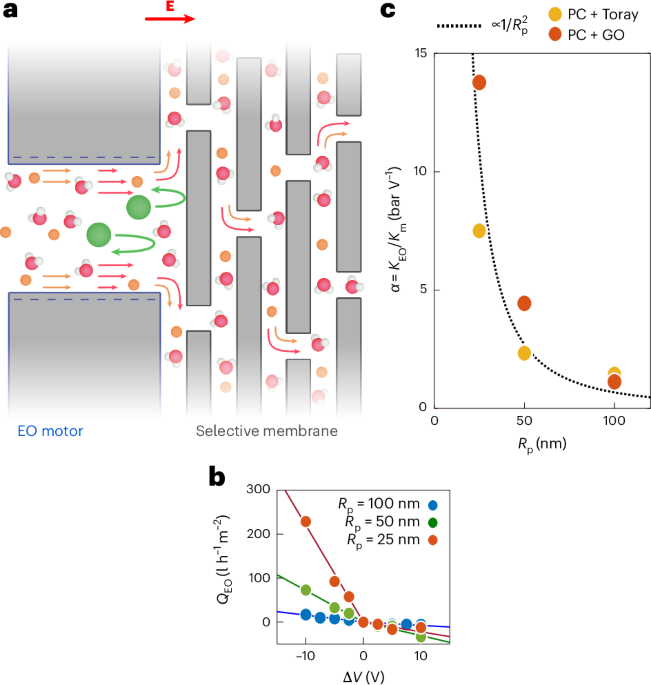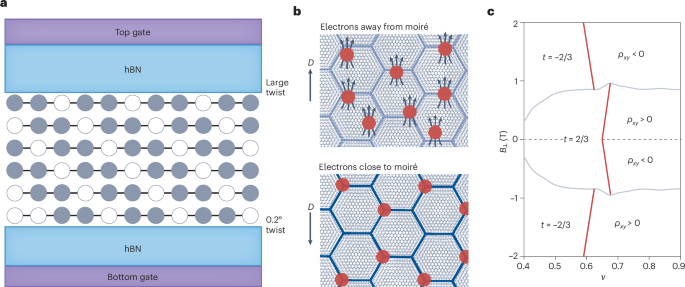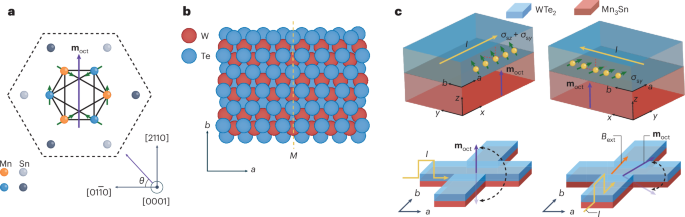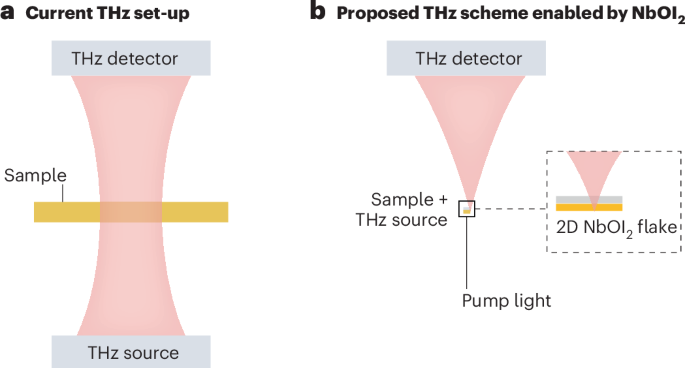
Turner, J. R. Intestinal mucosal barrier function in health and disease. Nat. Rev. Immunol. 9, 799–809 (2009).
Google Scholar
Duszyc, K. & Viasnoff, V. Mechanosensing and mechanotransduction at cell–cell junctions. Cold Spring Harb. Perspect. Biol. 10, a028761 (2018).
Google Scholar
Vasquez, C. G. & Martin, A. C. Force transmission in epithelial tissues. Dev. Dyn. 245, 361–371 (2016).
Google Scholar
Roan, E. & Waters, C. M. What do we know about mechanical strain in lung alveoli? Am. J. Physiol. Lung Cell. Mol. Physiol. 301, L625–L635 (2011).
Google Scholar
Foty, R. A. & Steinberg, M. S. The differential adhesion hypothesis: a direct evaluation. Dev. Biol. 278, 255–263 (2005).
Google Scholar
Honig, B. & Shapiro, L. Adhesion protein structure, molecular affinities, and principles of cell–cell recognition. Cell 181, 520–535 (2020).
Google Scholar
Brodland, G. W. The differential interfacial tension hypothesis (DITH): a comprehensive theory for the self-rearrangement of embryonic cells and tissues. J. Biomech. Eng. 124, 188–197 (2002).
Google Scholar
Maître, J.-L. et al. Adhesion functions in cell sorting by mechanically coupling the cortices of adhering cells. Science 338, 253–256 (2012).
Google Scholar
Winklbauer, R. Cell adhesion strength from cortical tension—an integration of concepts. J. Cell Sci. 128, 3687–3693 (2015).
Google Scholar
Chu, Y.-S. et al. Force measurements in E-cadherin-mediated cell doublets reveal rapid adhesion strengthened by actin cytoskeleton remodeling through Rac and Cdc42. J. Cell Biol. 167, 1183–1194 (2004).
Google Scholar
Atakhani, A., Bogdziewiez, L. & Verger, S. Characterising the mechanics of cell–cell adhesion in plants. Quant. Plant Biol. 3, e2 (2022).
Google Scholar
Sun, J.-Y. et al. Highly stretchable and tough hydrogels. Nature 489, 133–136 (2012).
Google Scholar
Yuk, H., Zhang, T., Lin, S., Parada, G. A. & Zhao, X. Tough bonding of hydrogels to diverse non-porous surfaces. Nat. Mater. 15, 190–196 (2016).
Google Scholar
Fedor-Chaiken, M., Hein, P. W., Stewart, J. C., Brackenbury, R. & Kinch, M. S. E-cadherin binding modulates EGF receptor activation. Cell Commun. Adhes. 10, 105–118 (2003).
Google Scholar
Engl, W., Arasi, B., Yap, L., Thiery, J. & Viasnoff, V. Actin dynamics modulate mechanosensitive immobilization of E-cadherin at adherens junctions. Nat. Cell Biol. 16, 584–591 (2014).
Google Scholar
Muhamed, I. et al. E-cadherin-mediated force transduction signals regulate global cell mechanics. J. Cell Sci. 129, 1843–1854 (2016).
Google Scholar
Fu, C., Arora, A., Engl, W., Sheetz, M. & Viasnoff, V. Cooperative regulation of adherens junction expansion through epidermal growth factor receptor activation. J. Cell Sci. 135, jcs258929 (2022).
Google Scholar
Fukata, M. & Kaibuchi, K. Rho-family GTPases in cadherin-mediated cell–cell adhesion. Nat. Rev. Mol. Cell Biol. 2, 887–897 (2001).
Google Scholar
Padmanabhan, A., Ong, H. T. & Zaidel-Bar, R. Non-junctional E-cadherin clusters regulate the actomyosin cortex in the C. elegans zygote. Curr. Biol. 27, 103–112 (2017).
Google Scholar
Hong, S., Troyanovsky, R. B. & Troyanovsky, S. M. Spontaneous assembly and active disassembly balance adherens junction homeostasis. Proc. Natl Acad. Sci. USA 107, 3528–3533 (2010).
Google Scholar
Engl, W., Arasi, B., Yap, L. L., Thiery, J. P. & Viasnoff, V. Actin dynamics modulate mechanosensitive immobilization of E-cadherin at adherens junctions. Nat. Cell Biol. 16, 587–594 (2014).
Google Scholar
Krendel, M., Zenke, F. T. & Bokoch, G. M. Nucleotide exchange factor GEF-H1 mediates cross-talk between microtubules and the actin cytoskeleton. Nat. Cell Biol. 4, 294–301 (2002).
Google Scholar
Ramirez Moreno, M. & Bulgakova, N. A. The cross-talk between EGFR and E-cadherin. Front. Cell Dev. Biol. 9, 828673 (2021).
Google Scholar
Proux-Gillardeaux, V. et al. Identification of a new regulation pathway of EGFR and E-cadherin dynamics. Sci. Rep. 11, 22705 (2021).
Google Scholar
Zuker, M. Mfold web server for nucleic acid folding and hybridization prediction. Nucleic Acids Res. 31, 3406–3415 (2003).
Google Scholar
Seifert, U. Rupture of multiple parallel molecular bonds under dynamic loading. Phys. Rev. Lett. 84, 2750–2753 (2000).
Google Scholar
Tong, J., Li, L., Ballermann, B. & Wang, Z. Phosphorylation and activation of RhoA by ERK in response to epidermal growth factor stimulation. PLoS ONE 11, e0147103 (2016).
Google Scholar
Kurokawa, K., Itoh, R. E., Yoshizaki, H., Nakamura, Y. O. T. & Matsuda, M. Coactivation of Rac1 and Cdc42 at lamellipodia and membrane ruffles induced by epidermal growth factor. Mol. Biol. Cell 15, 1003–1010 (2004).
Google Scholar
Ho, H.-Y. H. et al. Toca-1 mediates Cdc42-dependent actin nucleation by activating the N-WASP-WIP complex. Cell 118, 203–216 (2004).
Google Scholar